Redesigning API Batch Records Into a Clear, Controlled Lifecycle
Redesigning API Batch Records Into a Clear, Controlled Lifecycle
Redesigning API Batch Records Into a Clear, Controlled Lifecycle
About Project
About Project
About Project
After redesigning the BRMS for formulation teams, we extended the same structured, compliant workflow to BRMS-API (Active Pharmaceutical Ingredient) the module used across pharma plants to manage API Batch Manufacturing Records, including Production (BPR) and Cleaning Records.
It handles the entire API record lifecycle from drafting to issuance and obsolescence, keeping every batch traceable and compliant.
Our goal with BRMS-API was to create a clear, predictable, and fully controlled record management experience, helping plants maintain consistency, reduce manual effort, and streamline both production and cleaning documentation across all API facilities.
After redesigning the BRMS for formulation teams, we extended the same structured, compliant workflow to BRMS-API (Active Pharmaceutical Ingredient) the module used across pharma plants to manage API Batch Manufacturing Records, including Production (BPR) and Cleaning Records.
It handles the entire API record lifecycle from drafting to issuance and obsolescence, keeping every batch traceable and compliant.
Our goal with BRMS-API was to create a clear, predictable, and fully controlled record management experience, helping plants maintain consistency, reduce manual effort, and streamline both production and cleaning documentation across all API facilities.
About Project
Pharmaceutical
Team
Anush Reddy, S.Madhumala
Subscription Category
Quick win
Project start Year
August 2024
Core Business Challenges
Core Business Challenges
Core Business Challenges
Poor User Adoption Due to Outdated Experience
Poor User Adoption Due to Outdated Experience
Poor User Adoption Due to Outdated Experience
The outdated UI and unclear lifecycle made teams cross-check data constantly just to understand where an API batch stood.
The outdated UI and unclear lifecycle made teams cross-check data constantly just to understand where an API batch stood.
The outdated UI and unclear lifecycle made teams cross-check data constantly just to understand where an API batch stood.
Stuck in a Loop, Slowed by Inefficiency
Stuck in a Loop, Slowed by Inefficiency
Stuck in a Loop, Slowed by Inefficiency
Frequent support tickets arose from repetitive entry, scattered steps, and unclear stages slowing a process that must stay fast and compliant.
Frequent support tickets arose from repetitive entry, scattered steps, and unclear stages slowing a process that must stay fast and compliant.
Frequent support tickets arose from repetitive entry, scattered steps, and unclear stages slowing a process that must stay fast and compliant.
Heavy Dependence on Manual Coordination
Heavy Dependence on Manual Coordination
Teams depended on calls and offline checks to validate updates slowing approvals and increasing QA & Production workload.
Teams depended on calls and offline checks to validate updates slowing approvals and increasing QA & Production workload.
Heavy Dependence on Manual Coordination
Teams depended on calls and offline checks to validate updates slowing approvals and increasing QA & Production workload.
Our Approach
Our Approach
Our Approach
Mapping User Flows to Uncover Hidden Gaps
We analysed how API teams move through drafting, cleaning, equipment logs, and approvals. This helped us identify fragmented steps, manual coordination points, and unclear lifecycle transitions.
Mapping User Flows to Uncover Hidden Gaps
We analysed how API teams move through drafting, cleaning, equipment logs, and approvals. This helped us identify fragmented steps, manual coordination points, and unclear lifecycle transitions.
Mapping User Flows to Uncover Hidden Gaps
We analysed how API teams move through drafting, cleaning, equipment logs, and approvals. This helped us identify fragmented steps, manual coordination points, and unclear lifecycle transitions.
Designing API Batch Records Into a Streamlined Digital Flow
Designing API Batch Records Into a Streamlined Digital Flow
Designing API Batch Records Into a Streamlined Digital Flow
We extended BRMS to BRMS–API with a single, predictable flow for all API batch records. API steps, cleaning logs, and equipment records were standardised to reduce manual coordination. A simple BPR Cleaning Record switch keeps navigation easy, while clear version/status cues and role-based views show exactly what’s changed and what’s pending.
We extended BRMS to BRMS–API with a single, predictable flow for all API batch records. API steps, cleaning logs, and equipment records were standardised to reduce manual coordination. A simple BPR Cleaning Record switch keeps navigation easy, while clear version/status cues and role-based views show exactly what’s changed and what’s pending.




Navigation That Guides Every Step
Navigation That Guides Every Step
Navigation That Guides Every Step
We redesigned the side navigation into a clear, step-by-step flow Draft to Obsolete so users always know what comes next. Once a batch is drafted or approved, the system automatically guides them to the next action, removing confusion and manual tracking.
The BPR, Cleaning Record tab switch keeps both flows familiar but distinct, allowing users to move between processes without losing context.
We redesigned the side navigation into a clear, step-by-step flow Draft to Obsolete so users always know what comes next. Once a batch is drafted or approved, the system automatically guides them to the next action, removing confusion and manual tracking.
The BPR, Cleaning Record tab switch keeps both flows familiar but distinct, allowing users to move between processes without losing context.
We redesigned the side navigation into a clear, step-by-step flow Draft to Obsolete so users always know what comes next. Once a batch is drafted or approved, the system automatically guides them to the next action, removing confusion and manual tracking.
The BPR, Cleaning Record tab switch keeps both flows familiar but distinct, allowing users to move between processes without losing context.
Clear Information, Faster Action
Clear Information, Faster Action
Clear Information, Faster Action
We added key data Stage and Equipment directly into the main table so users no longer need to open records or switch screens to identify where a cleaning record belongs.
Everything is visible at a glance, reducing search time, avoiding mix-ups between stages, and helping QA and Production move approvals faster with full clarity.
We added key data Stage and Equipment directly into the main table so users no longer need to open records or switch screens to identify where a cleaning record belongs.
Everything is visible at a glance, reducing search time, avoiding mix-ups between stages, and helping QA and Production move approvals faster with full clarity.
We added key data Stage and Equipment directly into the main table so users no longer need to open records or switch screens to identify where a cleaning record belongs.
Everything is visible at a glance, reducing search time, avoiding mix-ups between stages, and helping QA and Production move approvals faster with full clarity.




Clarity & Traceability in Every Print Request
Clarity & Traceability in Every Print Request
Clarity & Traceability in Every Print Request
The new design makes additional page requests clear, accurate. Instead of typing page numbers in comments, users can now choose All Pages, Specific Pages, or a Page Range in one click.
Approvers instantly see the requested range, number of copies, and batch details reducing back-and-forth and ensuring every print request is verified quickly and without errors.
The new design makes additional page requests clear, accurate. Instead of typing page numbers in comments, users can now choose All Pages, Specific Pages, or a Page Range in one click.
Approvers instantly see the requested range, number of copies, and batch details reducing back-and-forth and ensuring every print request is verified quickly and without errors.
The new design makes additional page requests clear, accurate. Instead of typing page numbers in comments, users can now choose All Pages, Specific Pages, or a Page Range in one click.
Approvers instantly see the requested range, number of copies, and batch details reducing back-and-forth and ensuring every print request is verified quickly and without errors.
Visual Cues That Keep Workflows Crystal Clear
Visual Cues That Keep Workflows Crystal Clear
Visual Cues That Keep Workflows Crystal Clear
UI cues like status tags, icons, empty states, and comment cards make every step instantly understandable. Users now get notified on the actions , also see what’s pending or completed at a glance.
UI cues like status tags, icons, empty states, and comment cards make every step instantly understandable. Users now get notified on the actions , also see what’s pending or completed at a glance.










A Modern UI for Faster, Cleaner Batch Records
A Modern UI for Faster, Cleaner Batch Records
A Modern UI for Faster, Cleaner Batch Records
The new UI feels cleaner and more structured, with clear hierarchy and predictable layouts that help users move through every step quickly and confidently.
The new UI feels cleaner and more structured, with clear hierarchy and predictable layouts that help users move through every step quickly and confidently.



Results That Transformed Batch Manufacturing Lifecycle
Results That Transformed Batch Manufacturing Lifecycle
Results That Transformed Batch Manufacturing Lifecycle
The redesigned BMR-API system completely transformed how teams create and issue batch records. What was once a slow, document-heavy, and error-prone is now a guided, traceable process.
The redesigned BMR-API system completely transformed how teams create and issue batch records. What was once a slow, document-heavy, and error-prone is now a guided, traceable process.

Stronger Compliance & Traceability
Stronger Compliance & Traceability
Stronger Compliance & Traceability
Clear lifecycle states, version control, and audit-ready records ensured every API batch document change was traceable and compliant.
Clear lifecycle states, version control, and audit-ready records ensured every API batch document change was traceable and compliant.
Clear lifecycle states, version control, and audit-ready records ensured every API batch document change was traceable and compliant.

Faster API Batch Execution
Structured workflows and automated state transitions reduced manual checks, enabling faster movement from drafting to approval and issuance.

Reduced Follow-ups & Support Dependency
Role-based views and consistent documentation eliminated clarification loops, reducing reliance on QA support and manual verification.

Faster API Batch Execution
Faster API Batch Execution
Structured workflows and automated state transitions reduced manual checks, enabling faster movement from drafting to approval and issuance.
Structured workflows and automated state transitions reduced manual checks, enabling faster movement from drafting to approval and issuance.

Reduced Follow-ups & Support Dependency
Reduce Follow-ups & Support Dependency
Role-based views and consistent documentation eliminated clarification loops, reducing reliance on QA support and manual verification.
Role-based views and consistent documentation eliminated clarification loops, reducing reliance on QA support and manual verification.
Deep-Dive Into More System
Deep-Dive Into More System
Deep-Dive Into More System
Browse every optimised Software and explore how legacy systems became intuitive.
Browse every optimised Software and explore how legacy systems became intuitive.
Browse every optimised Software and explore how legacy systems became intuitive.
RCAI

Root Cause Analysis with Intelligence
LMS
Learning Management System
LIMS
Laboratory Information Management System
S & OP
Sales & Operations Planning
E-BMR
Batch Manufacturing Recall
WMPS

Warehouse Management System
DMS
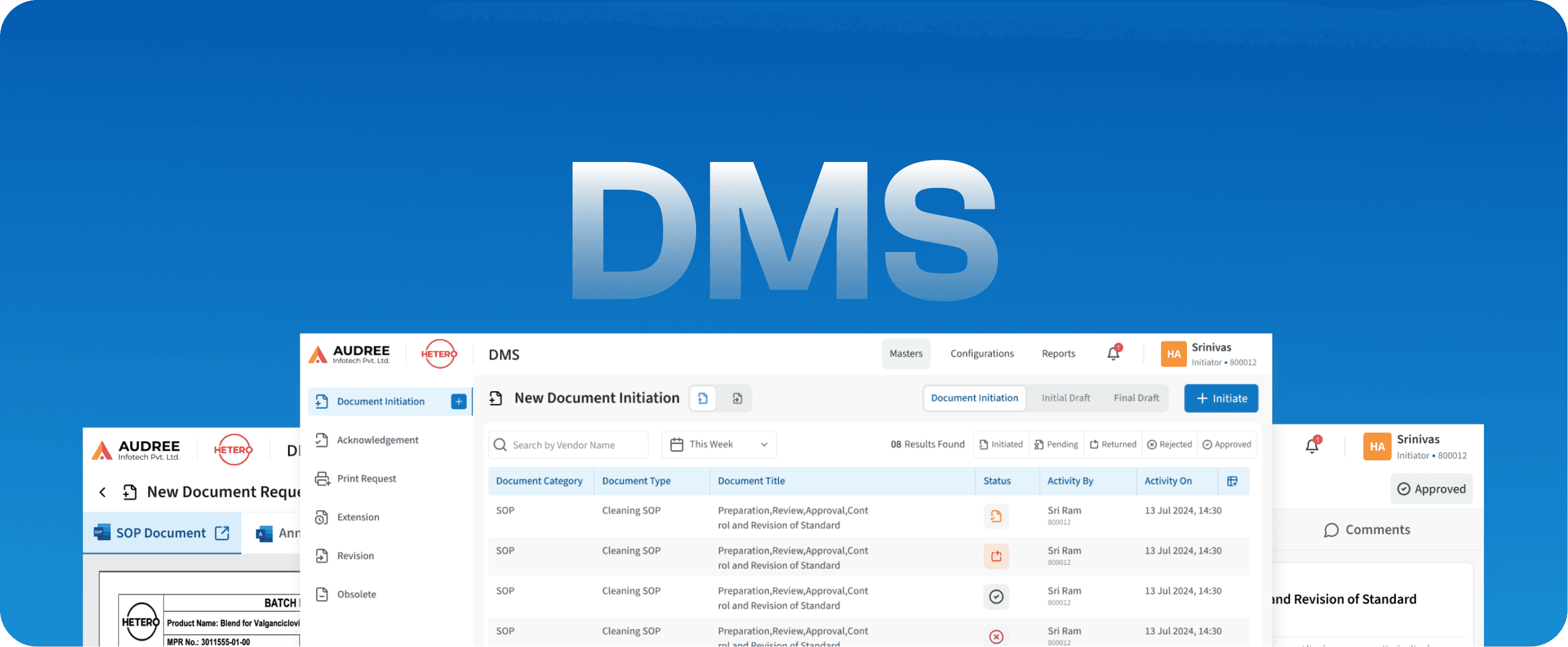
Document Management System
CAPA

Corrective And Preventive Actions
QAS

Quality Agreement System
Vendor Portal
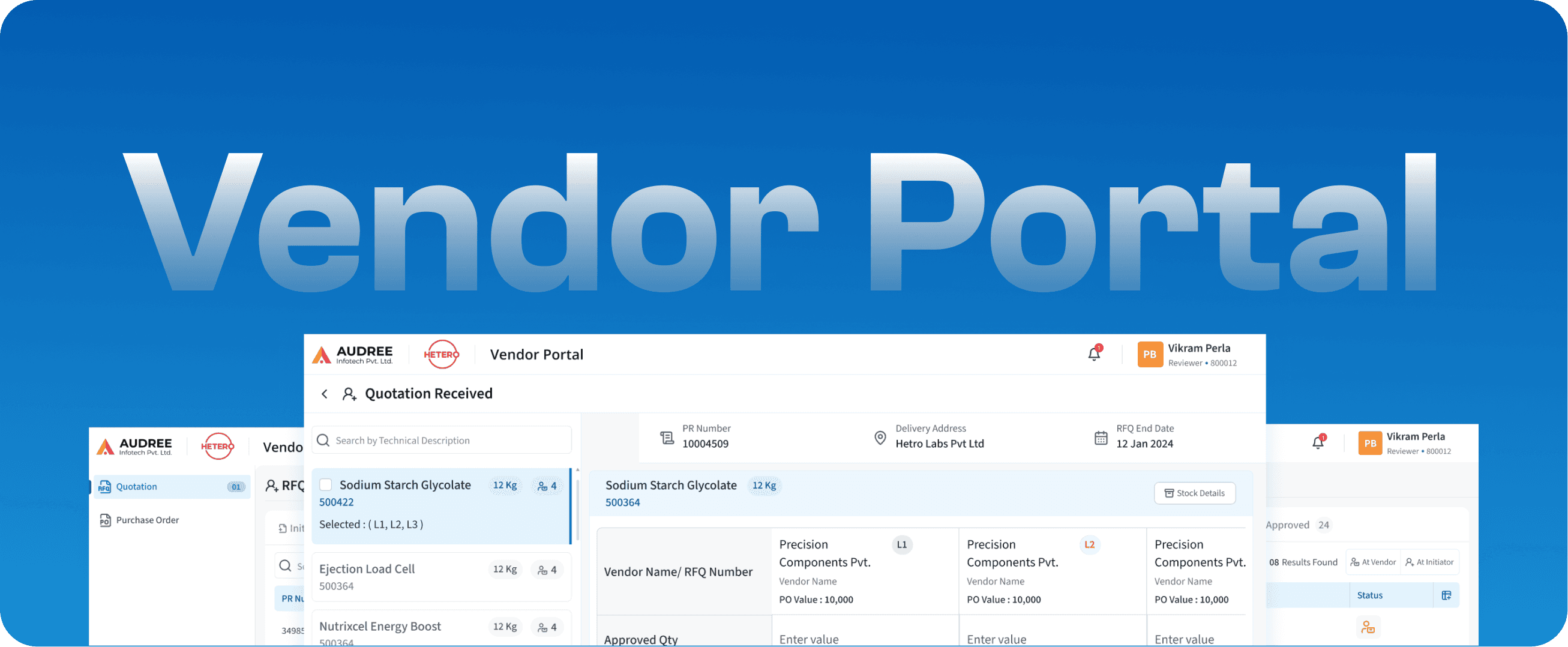
Vendor Management System
LIR-AER
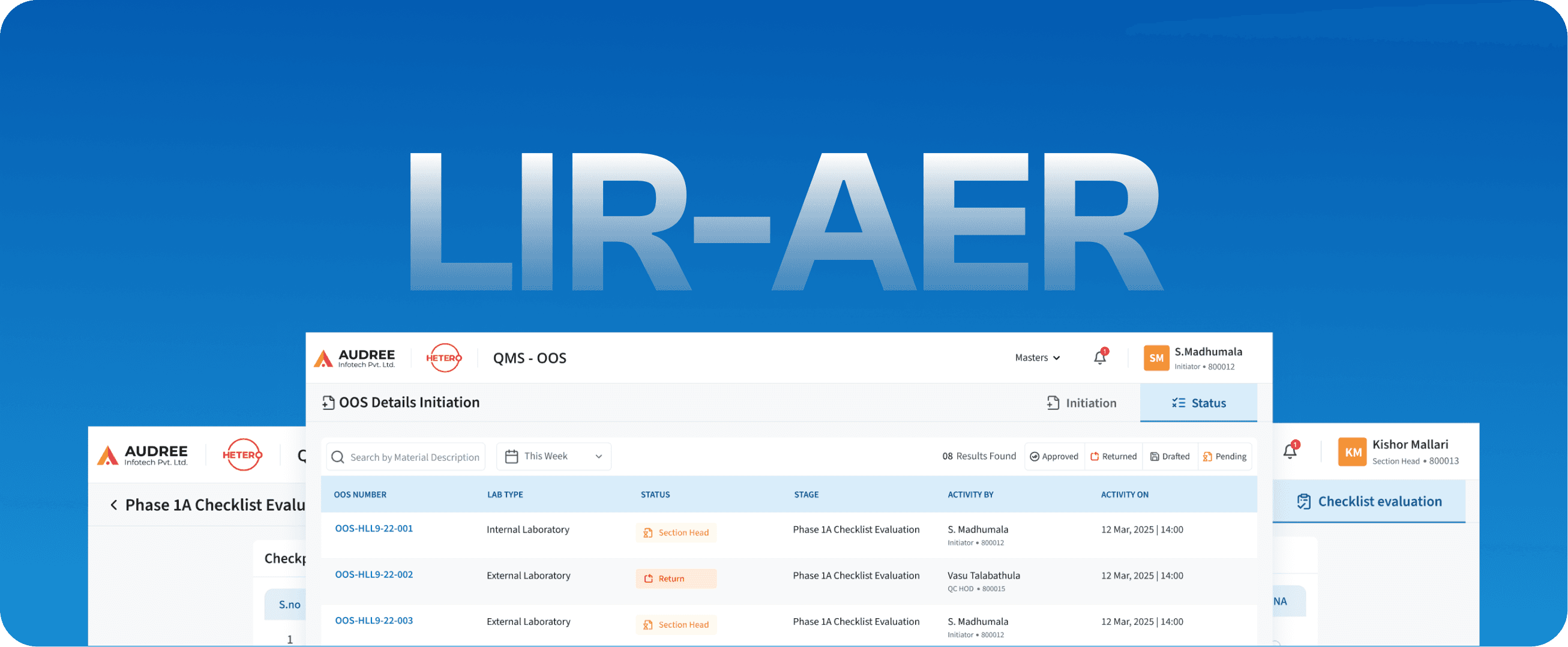
Laboratory Information Record
OOS
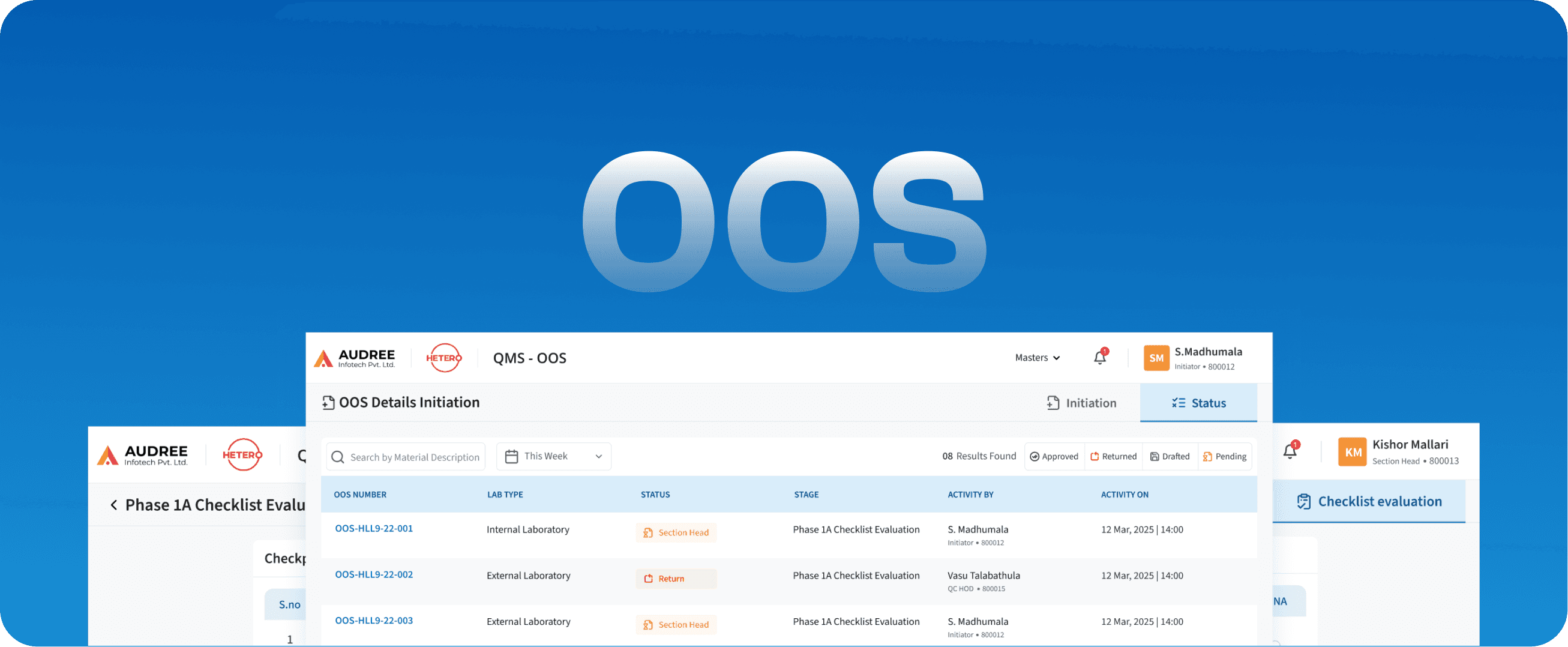
Out Of Specification
CMS
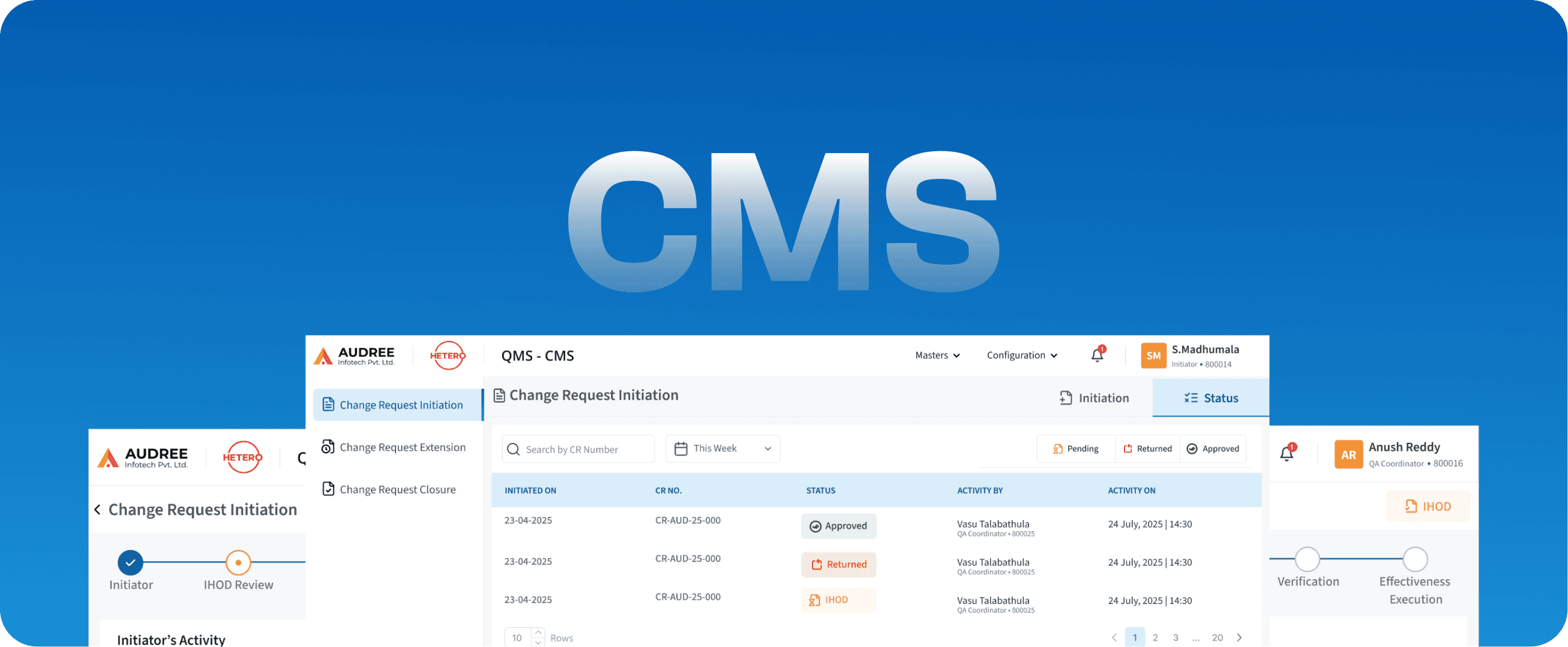
Change Management System
IMS
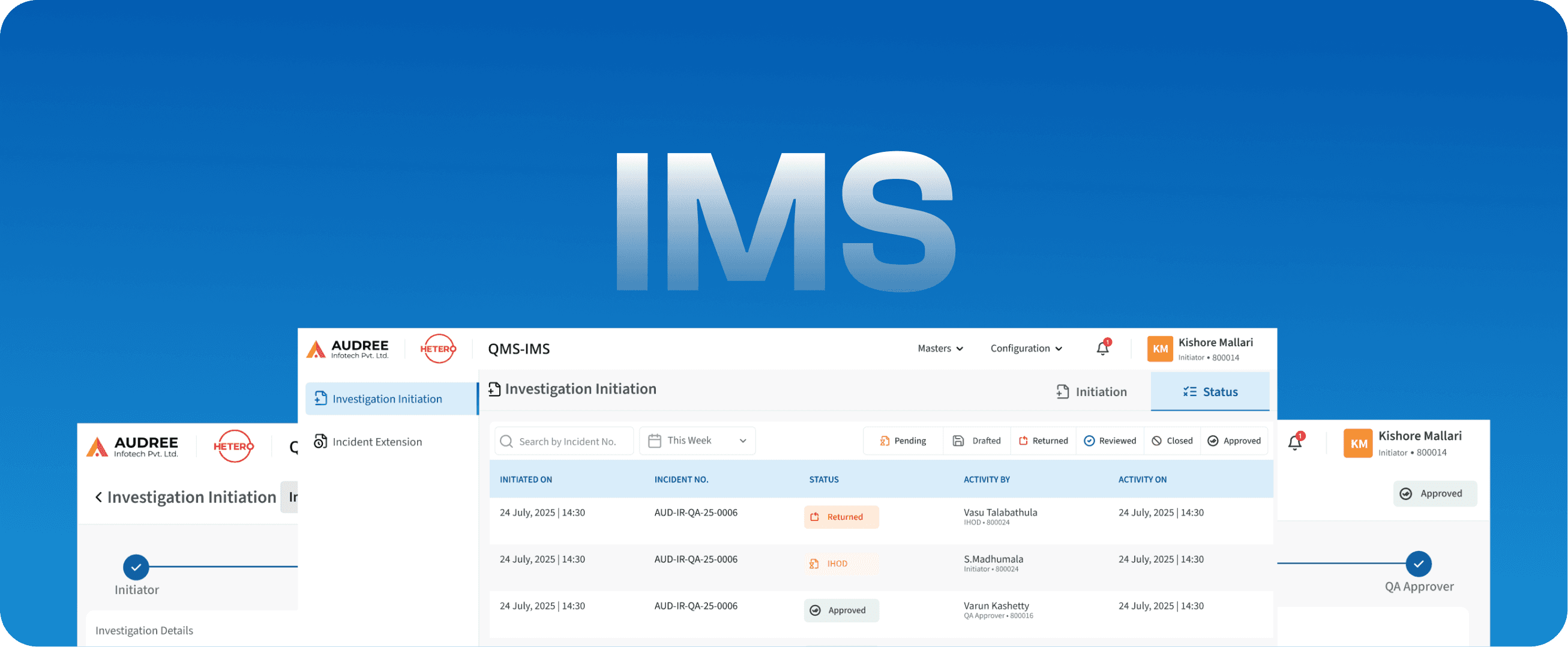
Incident Management System
BRMS-API
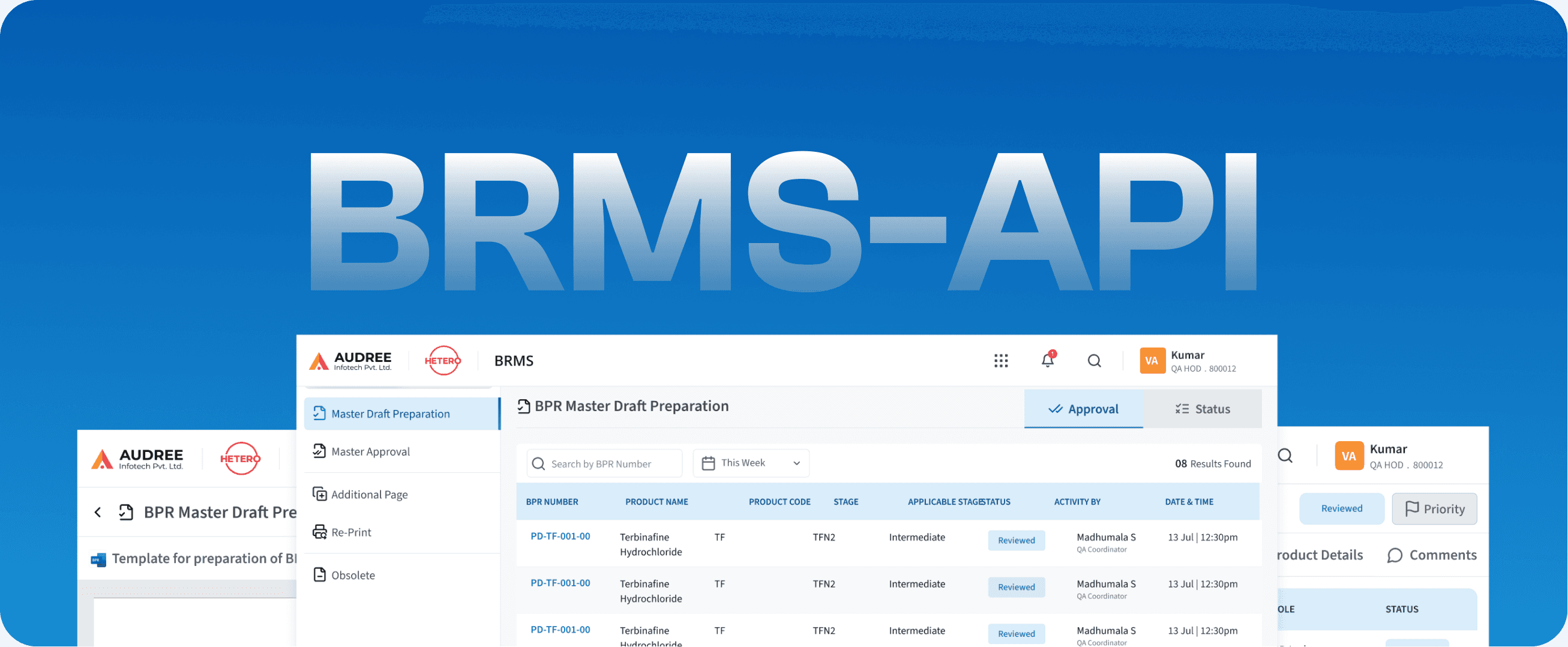
Batch Record- Active Pharmaceutical Ingredient
E-BRMS
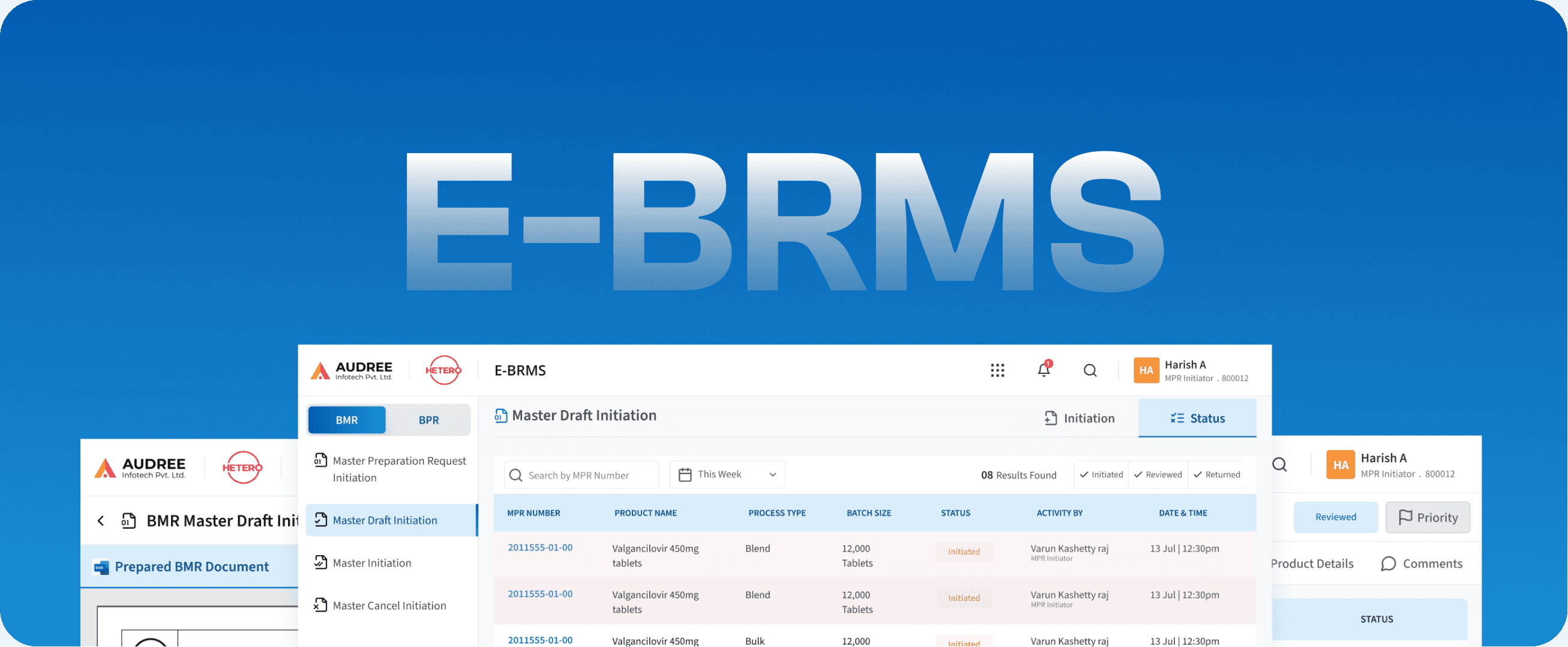
Batch Record Management System
RIMS
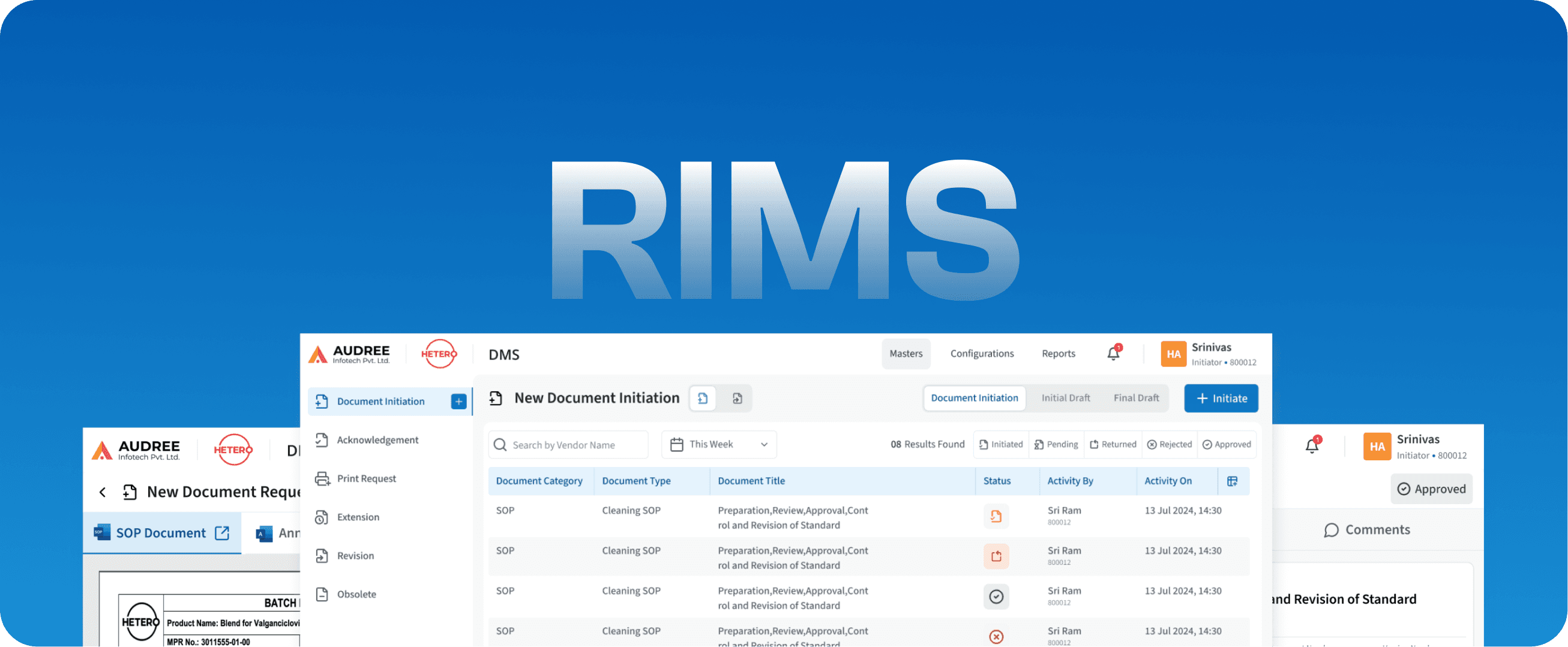
Regulatory Information Management System
APQR
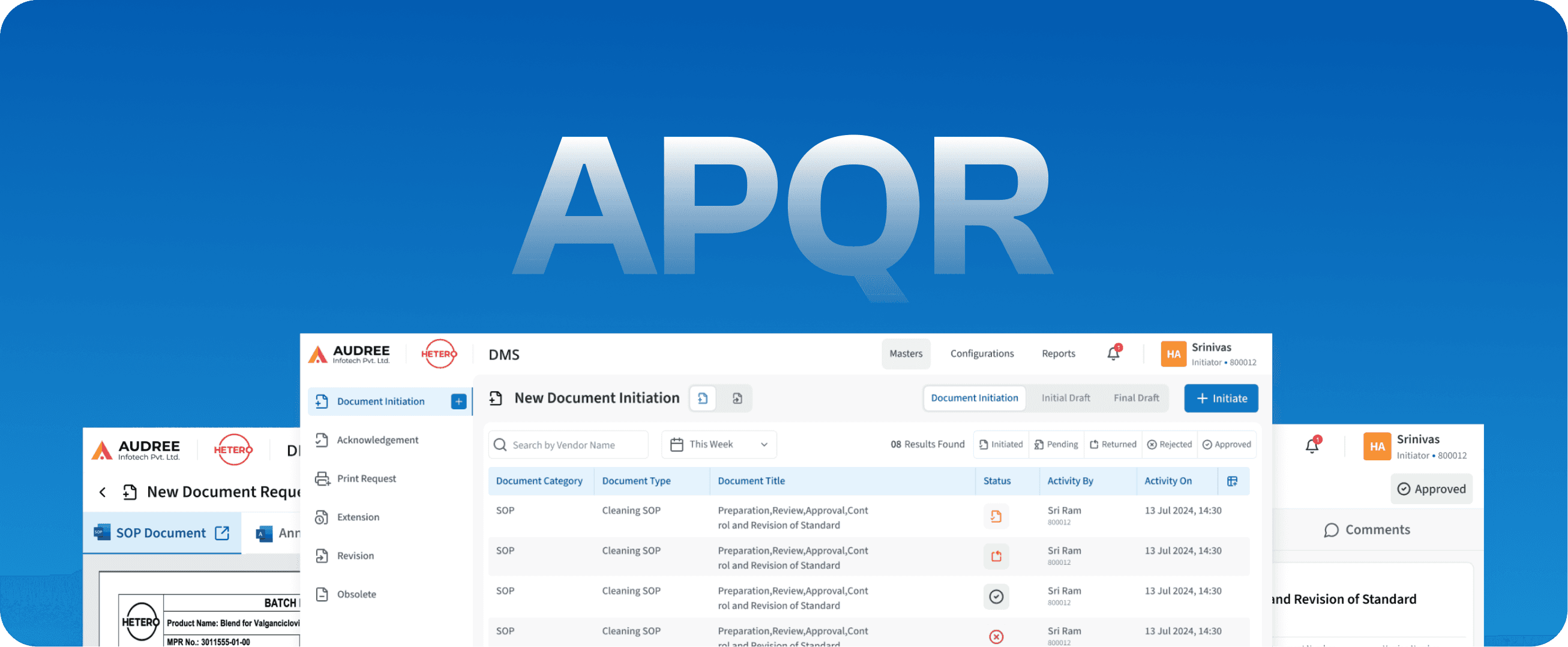
Annual Product Quality Review
RCAI

Root Cause Analysis with Intelligence
LMS

Learning Management System
LIMS

Laboratory Information Management System
S & OP

Sales & Operations Planning
E-BMR

Batch Manufacturing Recall
RIMS
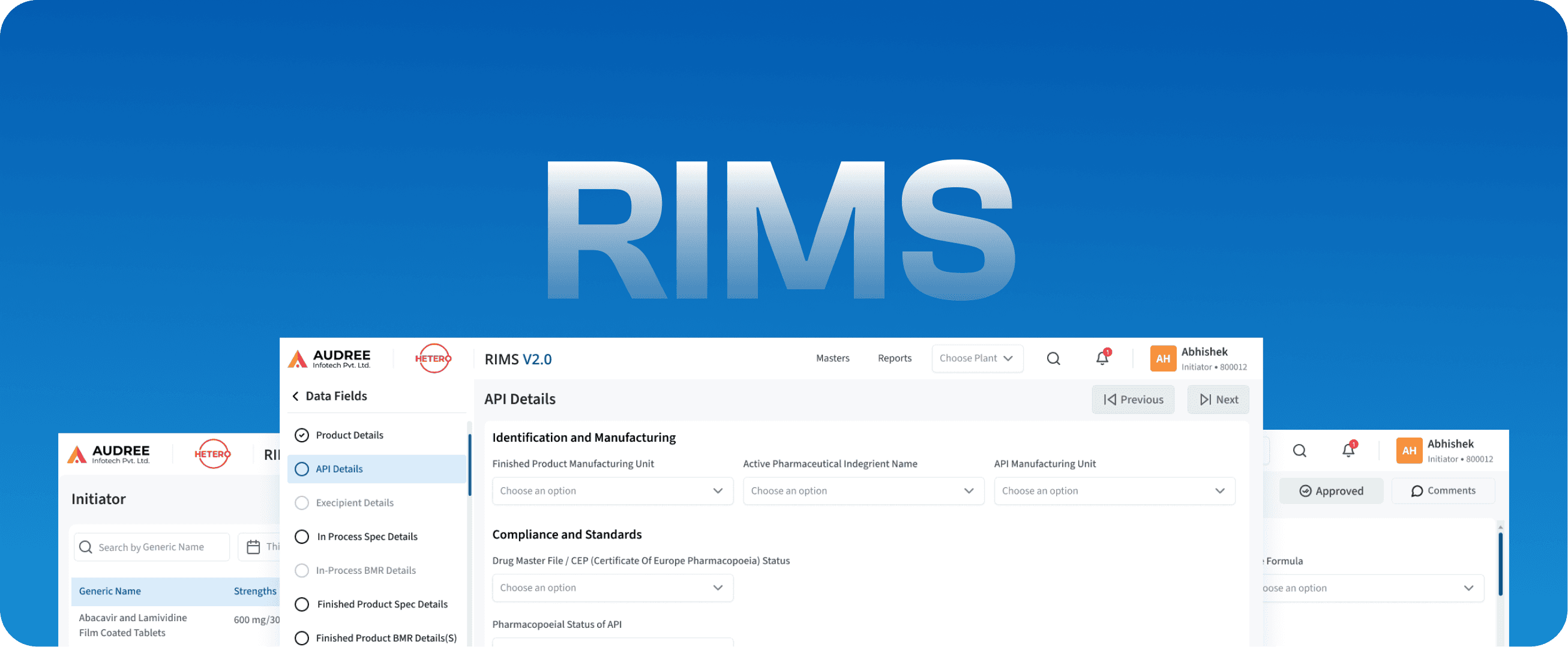
Regulatory Information Management Systems
IMS

Incident Management System
BRMS-API

Batch Record- Active Pharmaceutical Ingredient
E-BRMS

Batch Record Management System
APQR

Annual Product Quality Review
RIMS

Regulatory Information Management System
WMPS

Warehouse Management System
DMS

Document Management System
CMS

Change Management System
OOS

Out Of Specification
LIR-AER

Laboratory Information Record
Vendor Portal

Vendor Management System
QAS

Quality Agreement System
CAPA

Corrective And Preventive Actions

