Redesigned Pharmaceutical Softwares At Scale
Redesigned Pharmaceutical Softwares At Scale
Redesigned Pharmaceutical Softwares At Scale
Our Journey Optimizing
15+ Softwares in Partnership
with Audree
Our Journey Optimizing 15+ Softwares in Partnership with Audree
Our Journey Optimizing
15+ Softwares in Partnership with Audree
Audree Infotech, a leader in pharmaceutical software, partnered with us to first redesign their BRMS platform.
Our redesign delivered a dramatic leap in usability, efficiency, and adoption, leading Audree to entrust us with optimizing 15+ applications, all built on shared design principles and scalable systems, creating a unified, user-friendly, and faster to develop product ecosystem.
Audree Infotech, a leader in pharmaceutical software, partnered with us to first redesign their BRMS platform.
Our redesign delivered a dramatic leap in usability, efficiency, and adoption, leading Audree to entrust us with optimizing 15+ applications, all built on shared design principles and scalable systems, creating a unified, user-friendly, and faster to develop product ecosystem.
Business Challenges
Business Challenges
Business Challenges
Low Retention Across Modules
Low Retention Across Modules
Low Retention Across Modules
Teams reverted to Excel and manual logs because the UI was inconsistent and hard to follow leading to poor adoption.
Teams reverted to Excel and manual logs because the UI was inconsistent and hard to follow leading to poor adoption.
Teams reverted to Excel and manual logs because the UI was inconsistent and hard to follow leading to poor adoption.
Stuck in a Loop, Growth Stagnation
Stuck in a Loop, Growth Stagnation
Stuck in a Loop, Growth Stagnation
Usability gaps across systems kept teams stuck in support tickets and manual errors, leaving little room for innovation and slowing platform growth.
Usability gaps across systems kept teams stuck in support tickets and manual errors, leaving little room for innovation and slowing platform growth.
Usability gaps across systems kept teams stuck in support tickets and manual errors, leaving little room for innovation and slowing platform growth.
Heavy training Requirement
Heavy training Requirement
BRMS, LIMS, and DMS demanded frequent training because of complex workflows. Teams spent more time supporting users than improving or scaling the platforms.
BRMS, LIMS, and DMS demanded frequent training because of complex workflows. Teams spent more time supporting users than improving or scaling the platforms.
Heavy training Requirement
BRMS, LIMS, and DMS demanded frequent training because of complex workflows. Teams spent more time supporting users than improving or scaling the platforms.
Our Approach
Our Approach
Our Approach
User Flow
We mapped every user journey across pharma operations from document creation and batch approvals to issuance and tracking and identified friction points like redundant steps and unclear task transitions. These insights helped us streamline workflows for faster, error-free execution.
User Flow
We mapped every user journey across pharma operations from document creation and batch approvals to issuance and tracking and identified friction points like redundant steps and unclear task transitions. These insights helped us streamline workflows for faster, error-free execution.
User Flow
We mapped every user journey across pharma operations from document creation and batch approvals to issuance and tracking and identified friction points like redundant steps and unclear task transitions. These insights helped us streamline workflows for faster, error-free execution.
Observed Workflows Firsthand
Observed Workflows Firsthand
Observed Workflows Firsthand
Warehouse

Production

Laboratory

Warehouse

Production

Laboratory

Warehouse

On-site warehouse observations revealed real workflow constraints, shaping software aligned with material handling.
View Case study
Production

On-site production observations helped align the system with real manufacturing steps, validations, and approvals.
View Case study
Laboratory

Lab workflows were observed on-site to align the system with real testing, review, and compliance practices.
View Case study
Observed Workflows Across Software
Observed Workflows Across Software
Observed Workflows Across Software



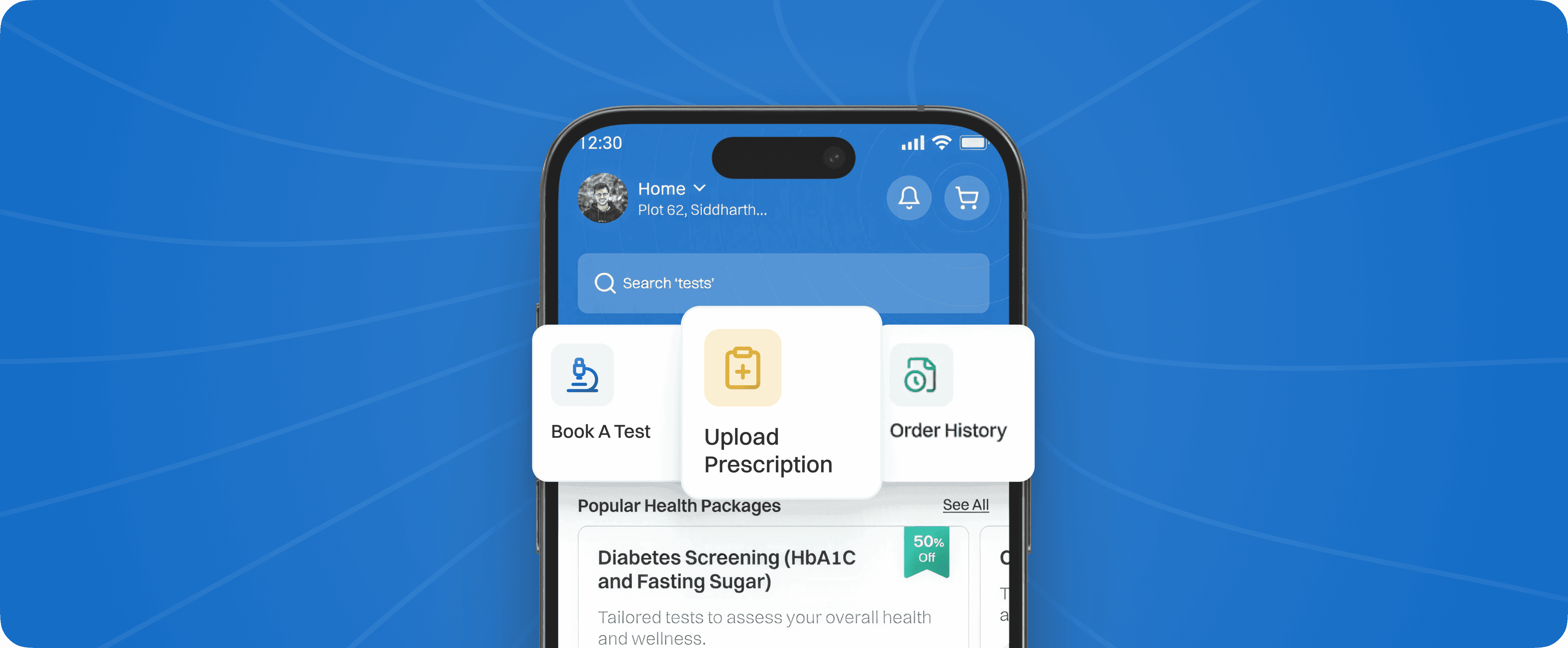
We redesigned BRMS to match real shop-floor workflows simplifying indications, digitizing paper-heavy steps, and making execution effortless for operators.
We redesigned BRMS to match real shop-floor workflows simplifying indications, digitizing paper-heavy steps, and making execution effortless for operators.
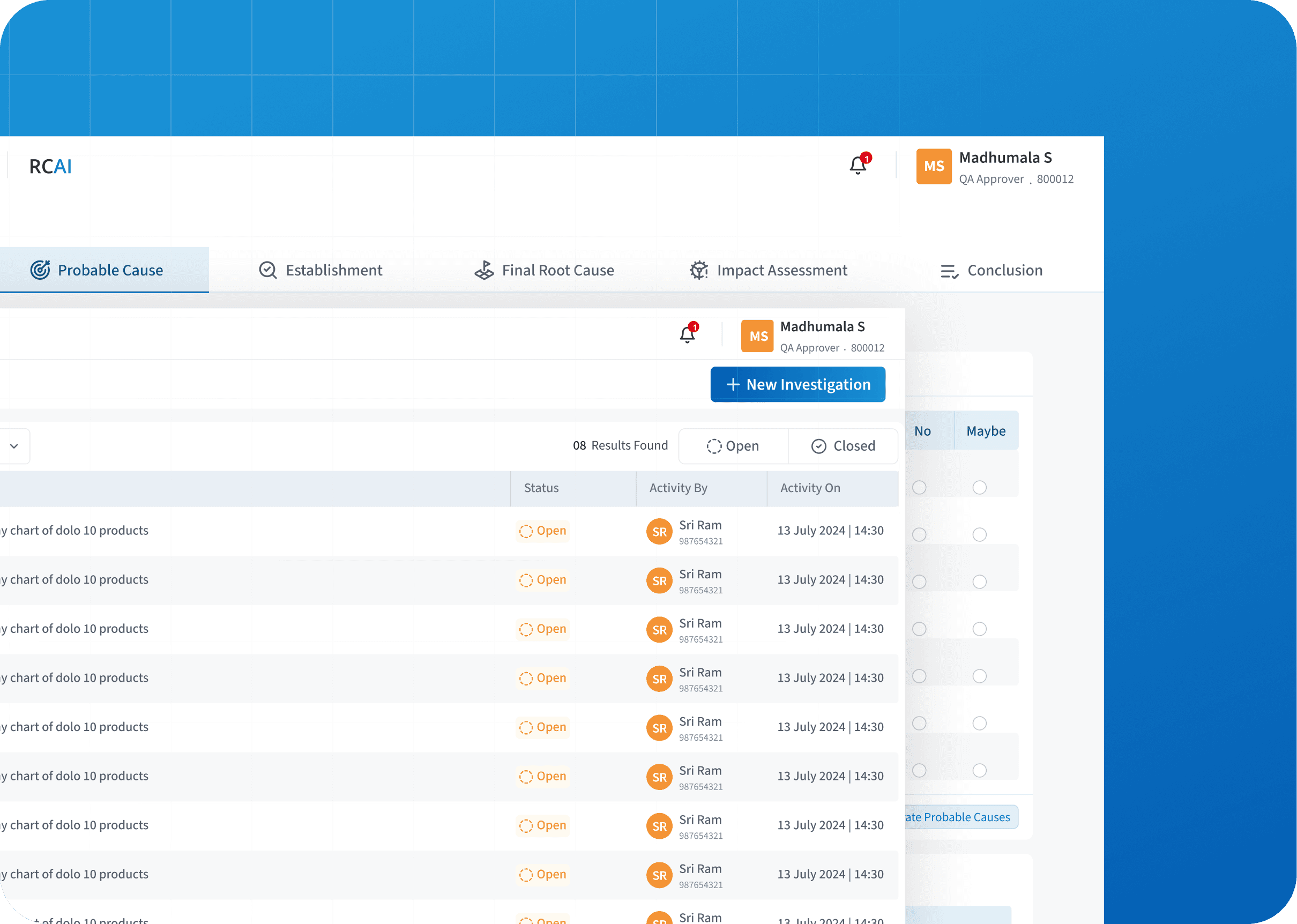


We simplified RCAI by structuring steps, evidence, and guiding users through clear assessments reducing errors and speeding up investigations.
We simplified RCAI by structuring steps, evidence, and guiding users through clear assessments reducing errors and speeding up investigations.
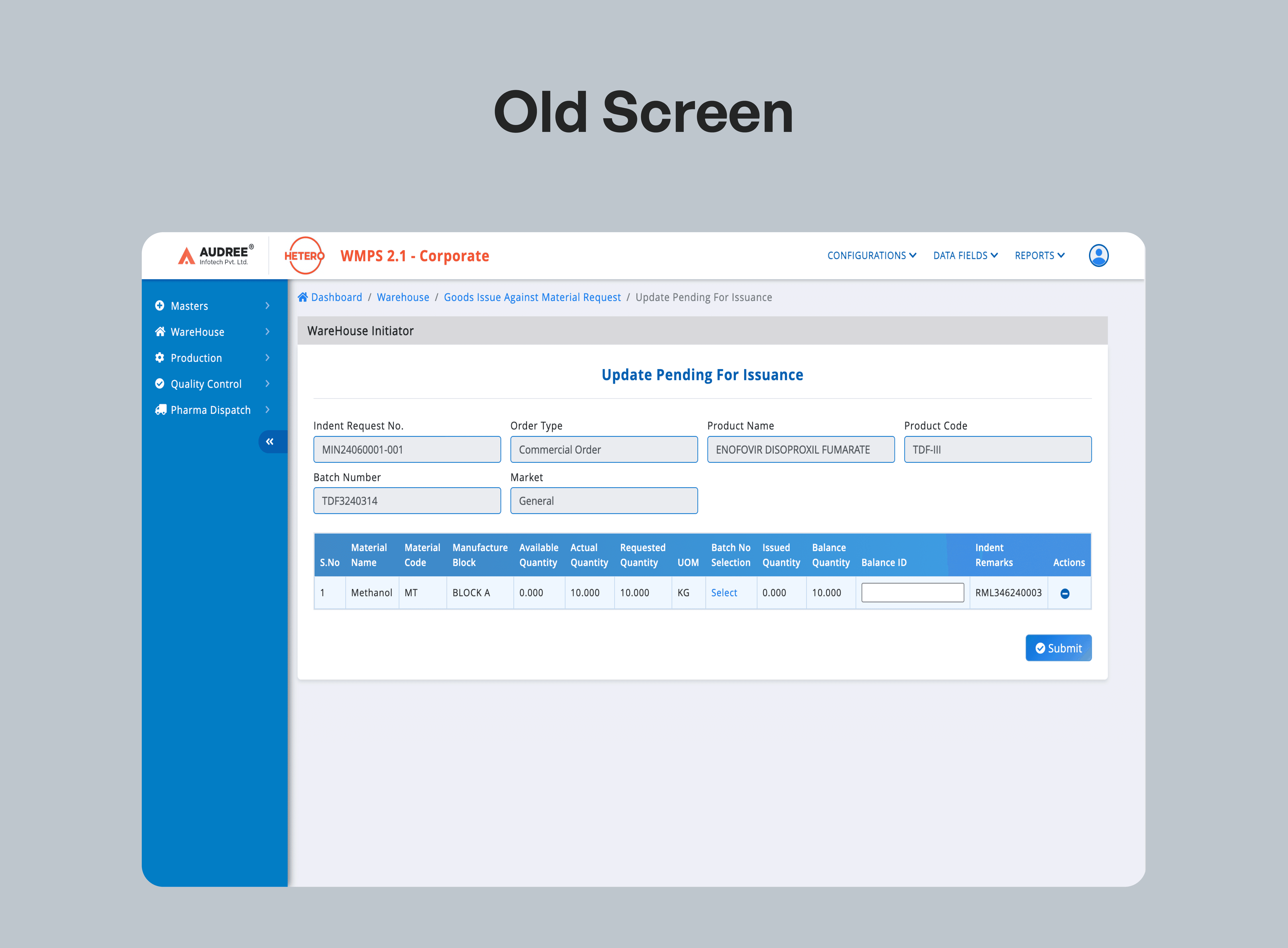
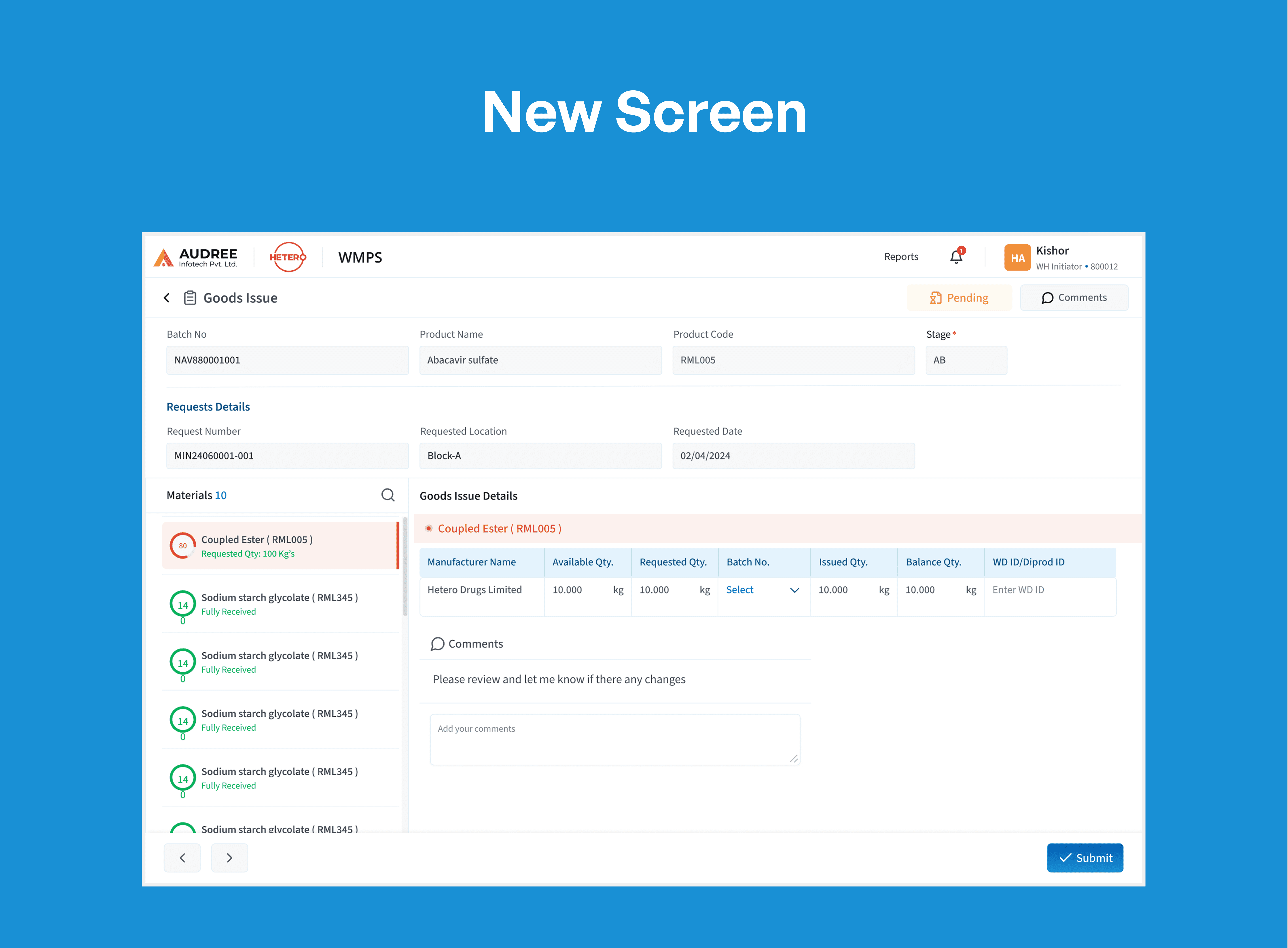
Adaptable Components Across Applications for Consistent Clarity
Adaptable Components Across Applications for Consistent Clarity
Adaptable Components Across Applications for Consistent Clarity
We designed a set of reusable UI components that not only unified the look and feel across 15+ pharma applications but also solved critical UX challenges.
We designed a set of reusable UI components that not only unified the look and feel across 15+ pharma applications but also solved critical UX challenges.
We designed a set of reusable UI components that not only unified the look and feel across 15+ pharma applications but also solved critical UX challenges.
Side Navigation

Side Navigation

Side Navigation

A cleaner menu, tab switching replaced the cluttered old navigation making task access faster and effortless.
A cleaner menu, tab switching replaced the cluttered old navigation making task access faster and effortless.
Comments Thread
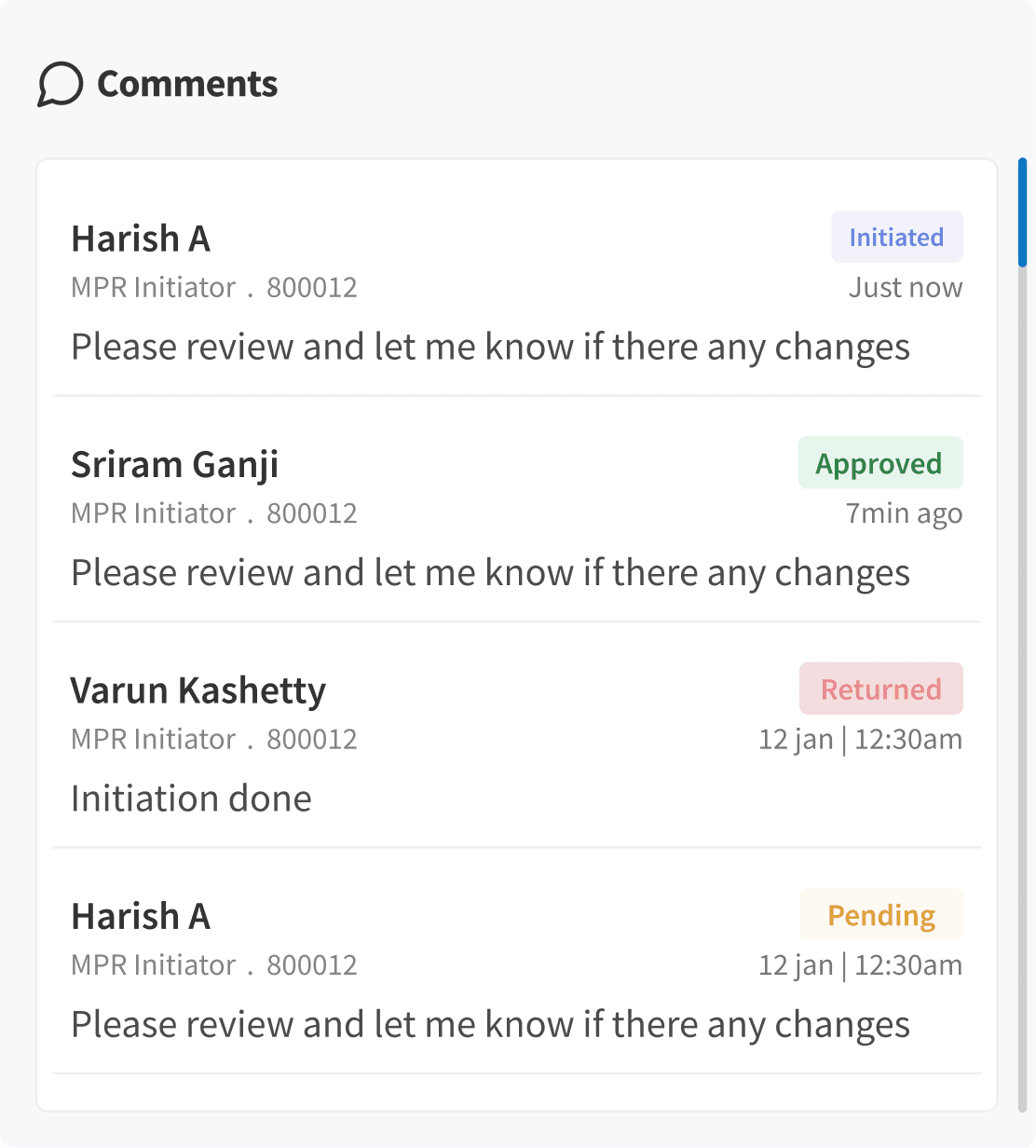
Comments Thread

Comments Thread

We redesigned the cluttered comment list into a clean, colour-coded thread that improves readability and speeds up review and approval cycles.
We redesigned the cluttered comment list into a clean, colour-coded thread that improves readability and speeds up review and approval cycles.
Product Details
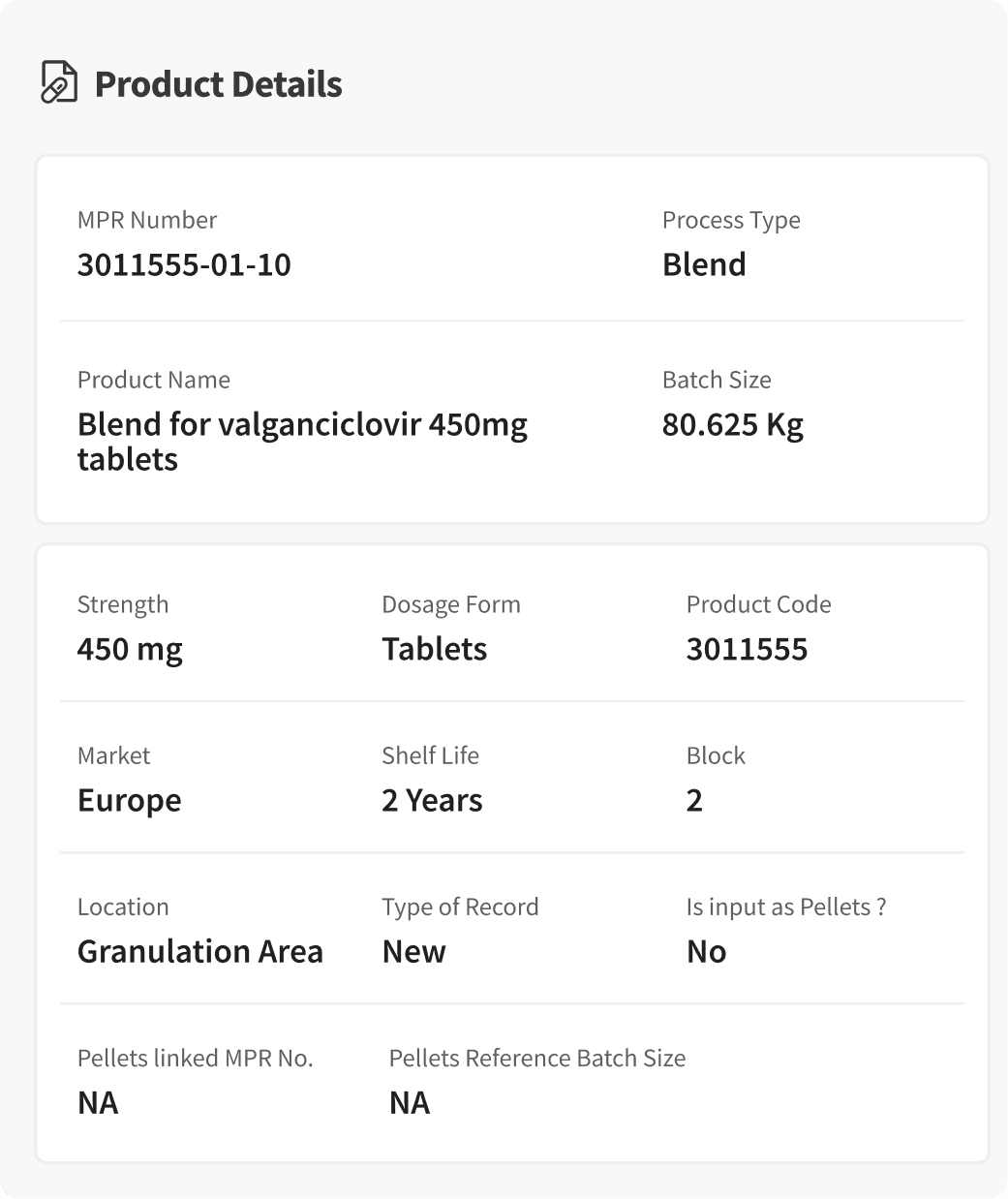
Product Details

Product Details

We simplified the dense, text-heavy product view into a clear, well-grouped layout, making critical information easier to scan and act on.
We simplified the dense, text-heavy product view into a clear, well-grouped layout, making critical information easier to scan and act on.
Table Componenet
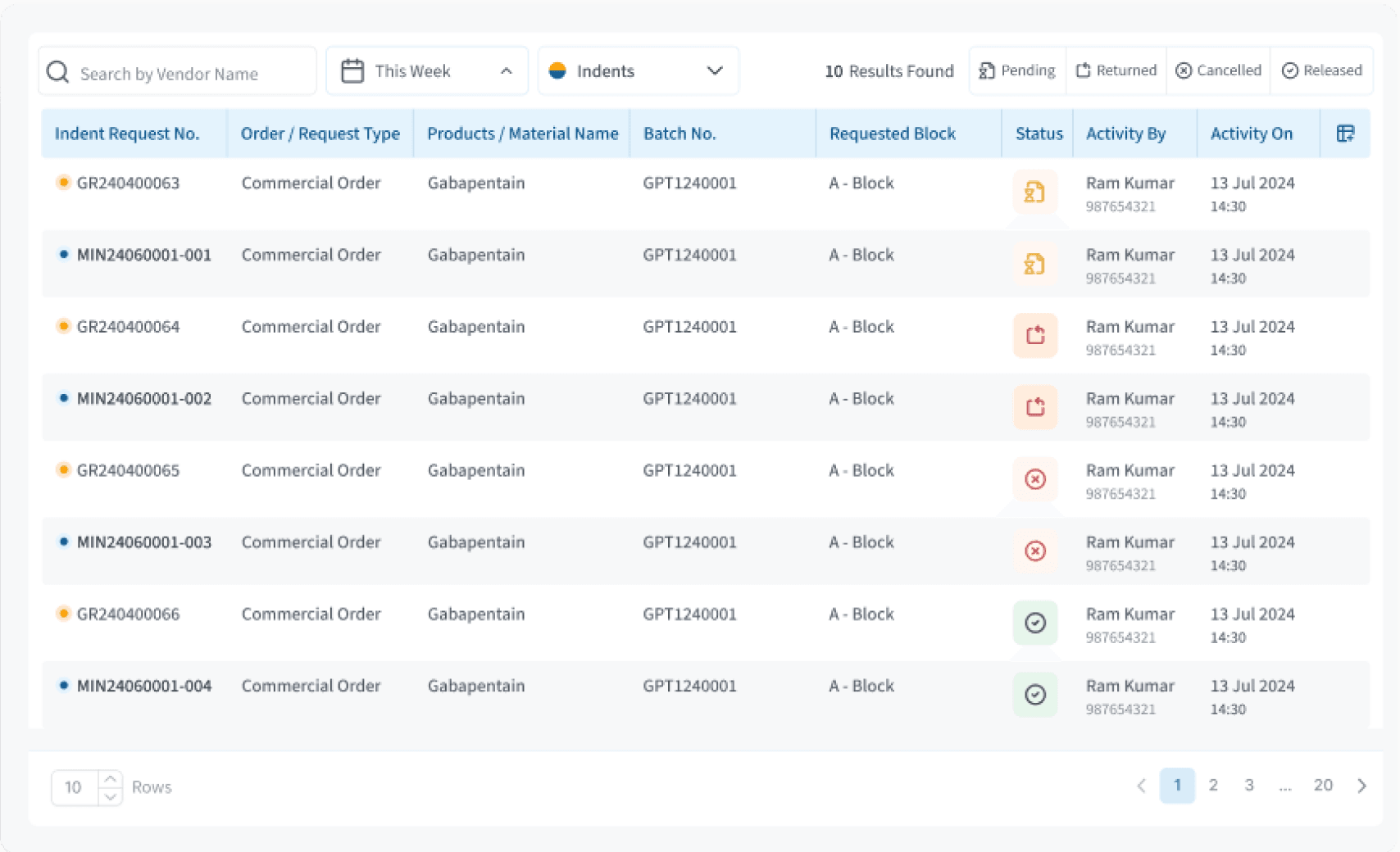
Table Componenet

Table Componenet

We reduced cluttered, data-heavy tables into a clean, scannable layout showing only what matters and colour-coded statuses for quick decisions.
We reduced cluttered, data-heavy tables into a clean, scannable layout showing only what matters and colour-coded statuses for quick decisions.
One System, Adaptable across multiple applications


Empty state
Status Tags
Created
Reviewed
Returned
Drafted
Rejected
Blocked
Approved
Pending
01
Design
System

One System, Adaptable across multiple applications


Empty state
Status Tags
Created
Reviewed
Returned
Drafted
Rejected
Blocked
Approved
Pending
01
Design
System

One System, Adaptable across multiple applications


Empty state
01
Design
System
Status Tags
Created
Reviewed
Returned
Drafted
Rejected
Blocked
Approved
Pending

Visual Consistency Across the Ecosystem
Visual Consistency Across the Ecosystem
Visual Consistency Across the Ecosystem
Colours and typography were selected based on pharma guidelines. We chose Source Sans 3 for its high legibility and compact letterforms ideal for text-heavy screens ensuring clarity, compliance.
We designed a set of reusable UI components that not only unified the look and feel across 15+ pharma applications but also solved critical UX challenges.
Colours and typography were selected based on pharma guidelines. We chose Source Sans 3 for its high legibility and compact letterforms ideal for text-heavy screens ensuring clarity, compliance.




Identical Interfaces Across Applications for Clarity and Efficiency
Identical Interfaces Across Applications for Clarity and Efficiency
Identical Interfaces Across Applications for Clarity and Efficiency
Deep-Dive Into Each System
Deep-Dive Into Each System
Deep-Dive Into Each System
Browse every optimised Software and explore how legacy systems became intuitive.
Browse every optimised Software and explore how legacy systems became intuitive.
RCAI

Root Cause Analysis with Intelligence
LMS
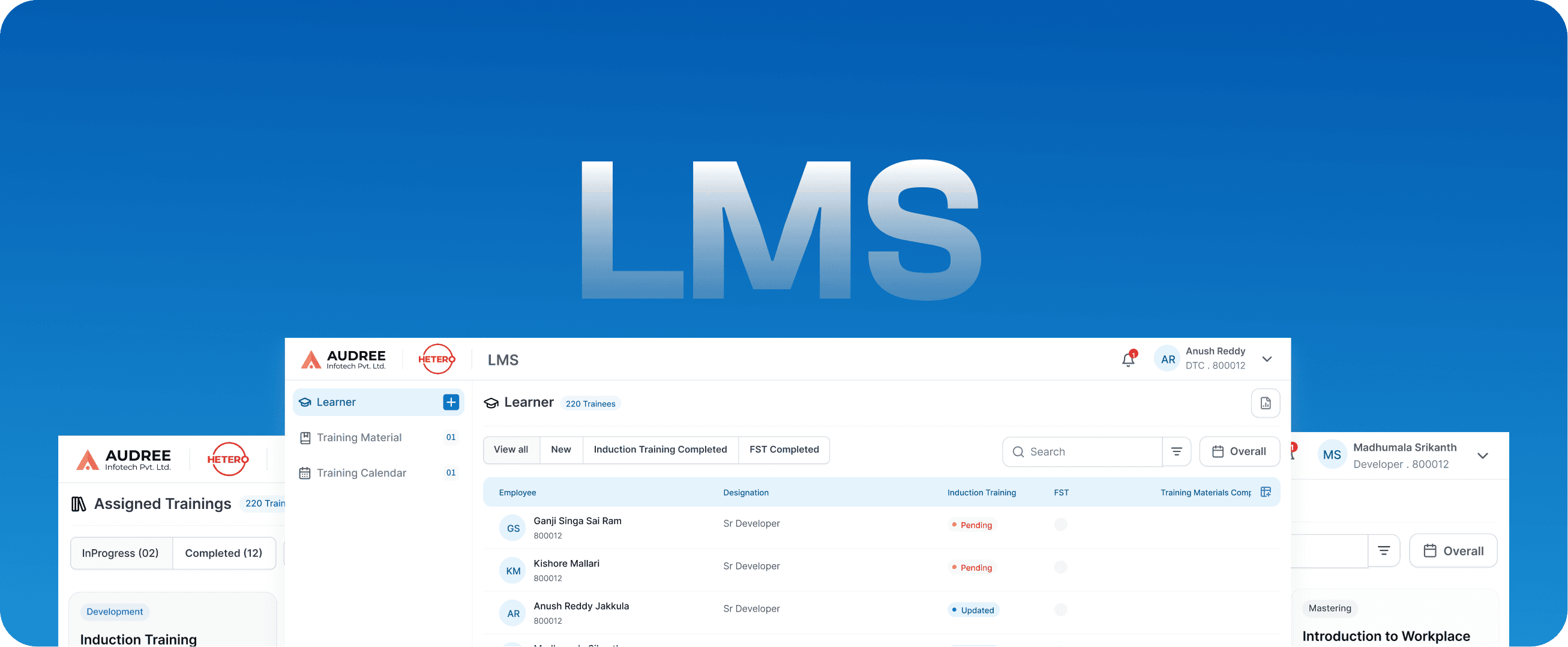
Learning Management System
LIMS
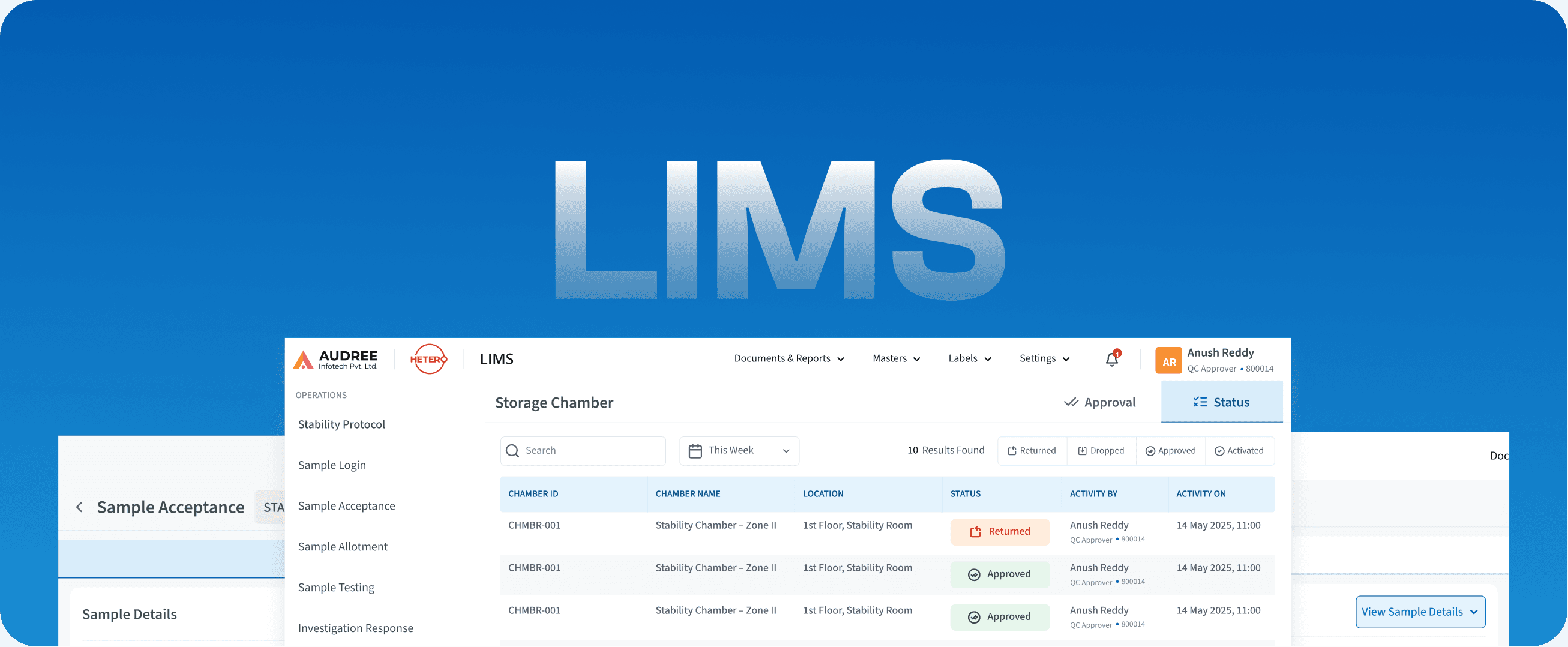
Laboratory Information Management System
S & OP
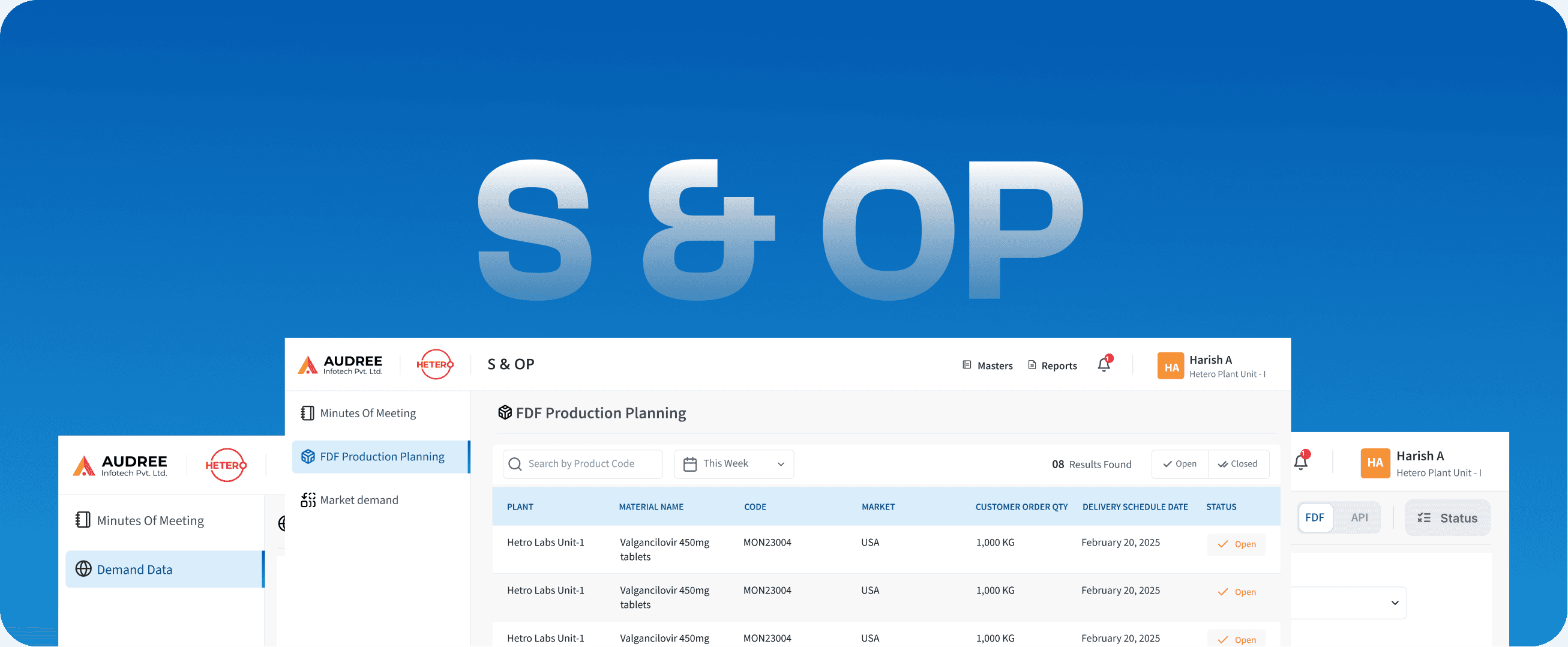
Sales & Operations Planning
E-BMR
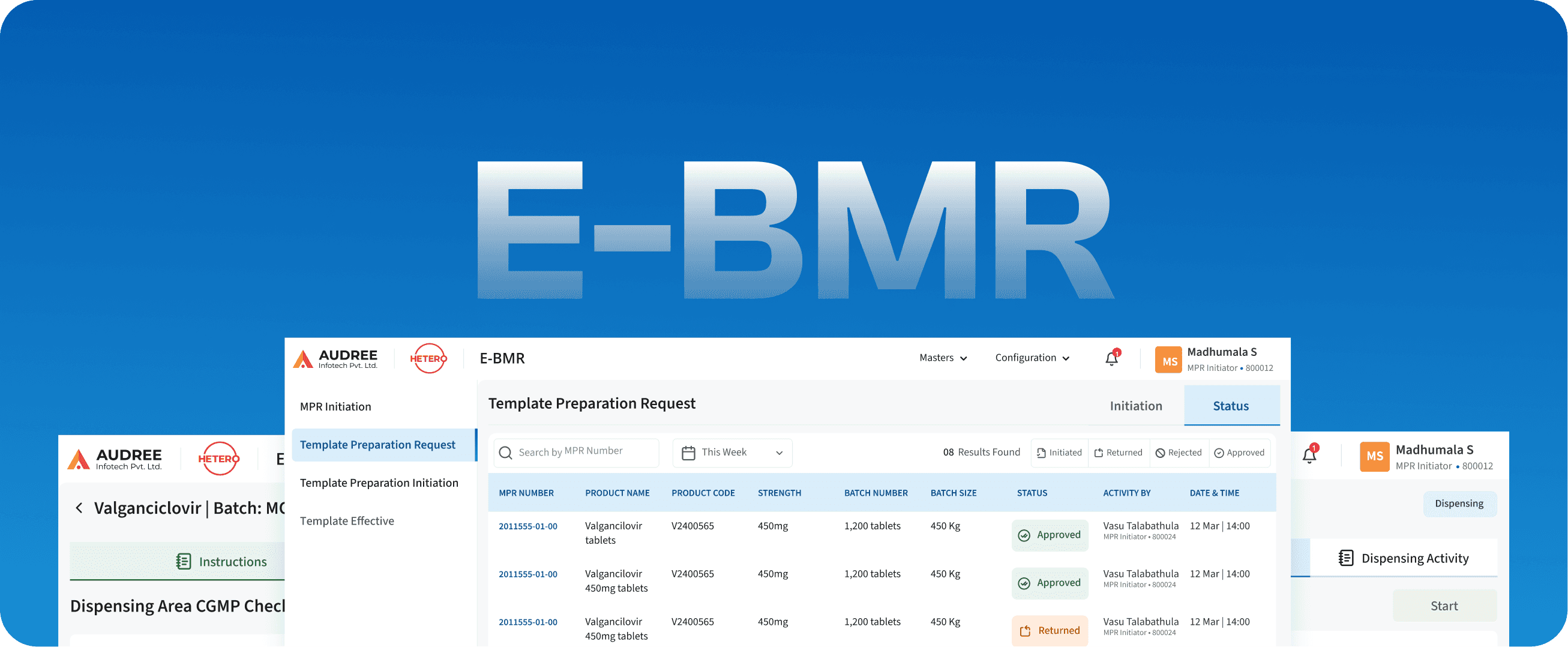
Batch Manufacturing Recall
WMPS

Warehouse Management System
DMS
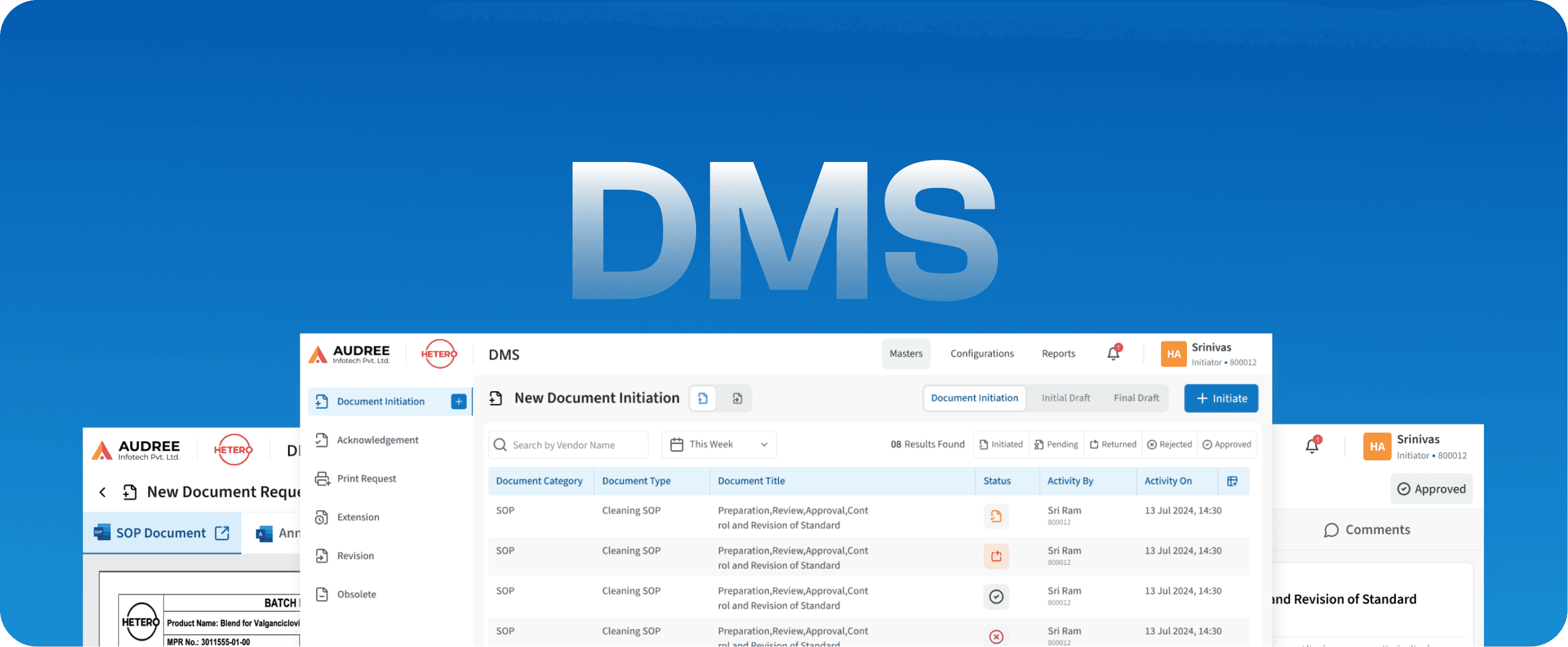
Document Management System
CAPA

Corrective And Preventive Actions
QAS

Quality Agreement System
Vendor Portal
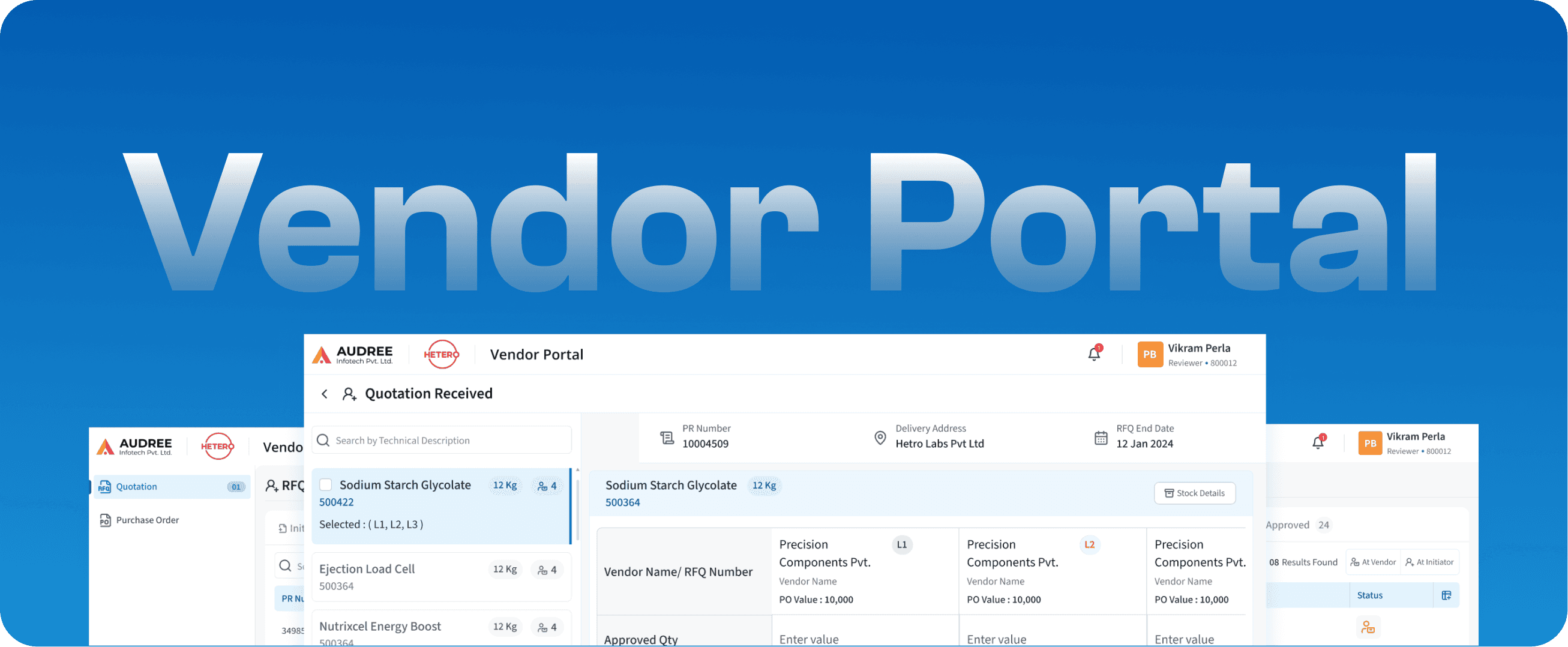
Vendor Management System
LIR-AER
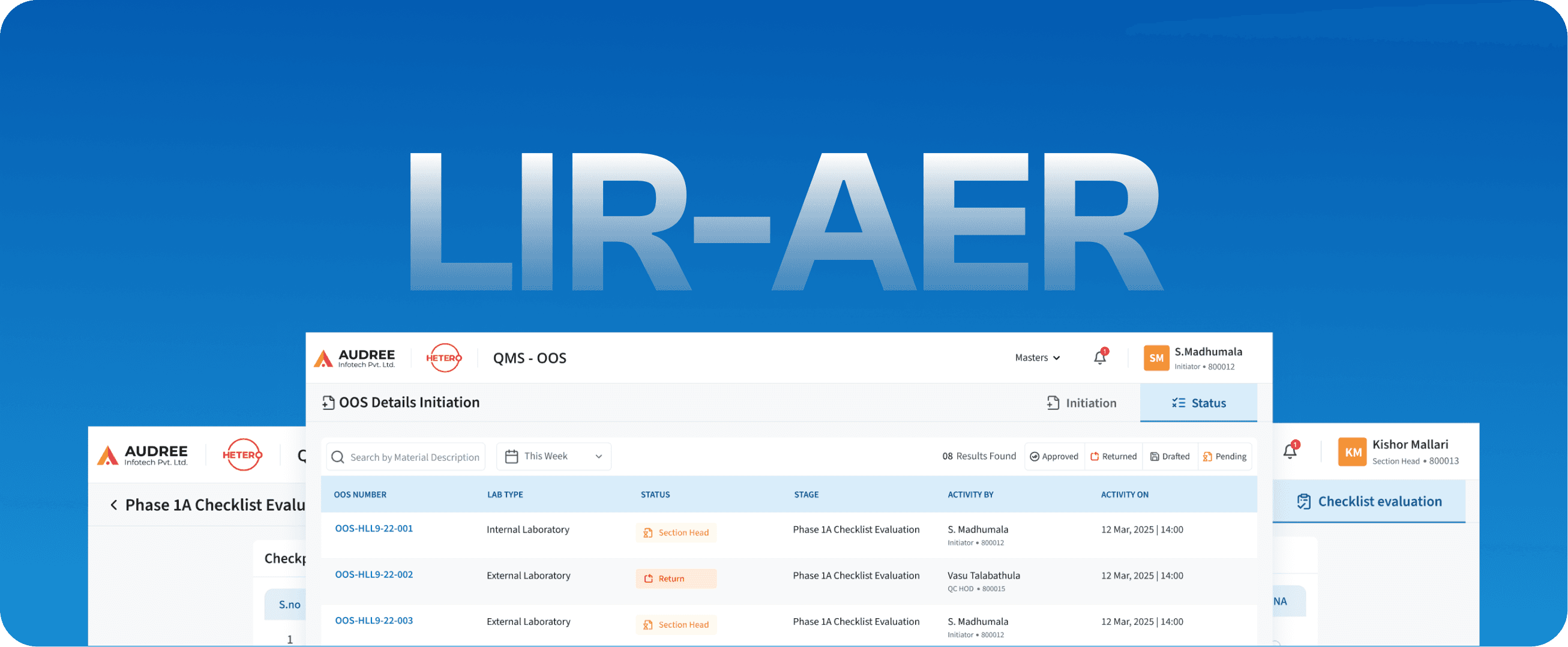
Laboratory Information Record
OOS
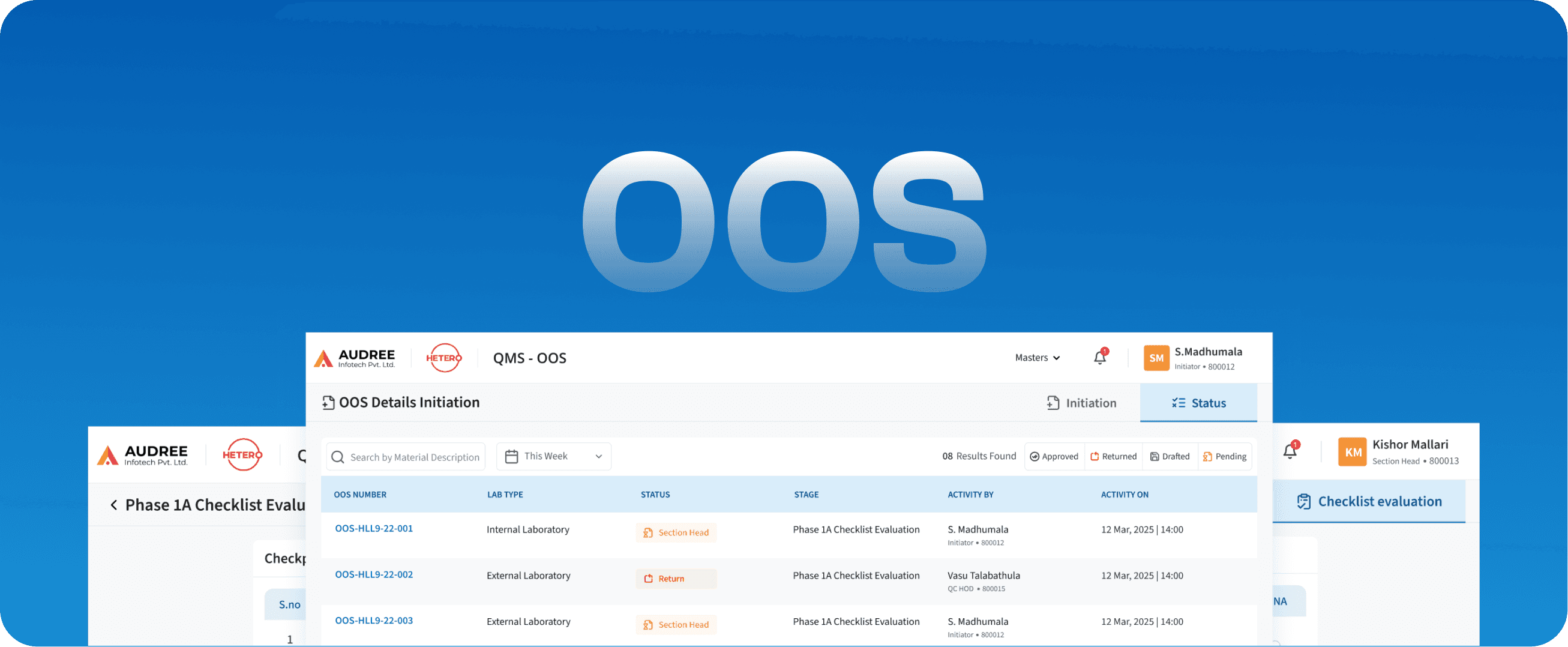
Out Of Specification
CMS
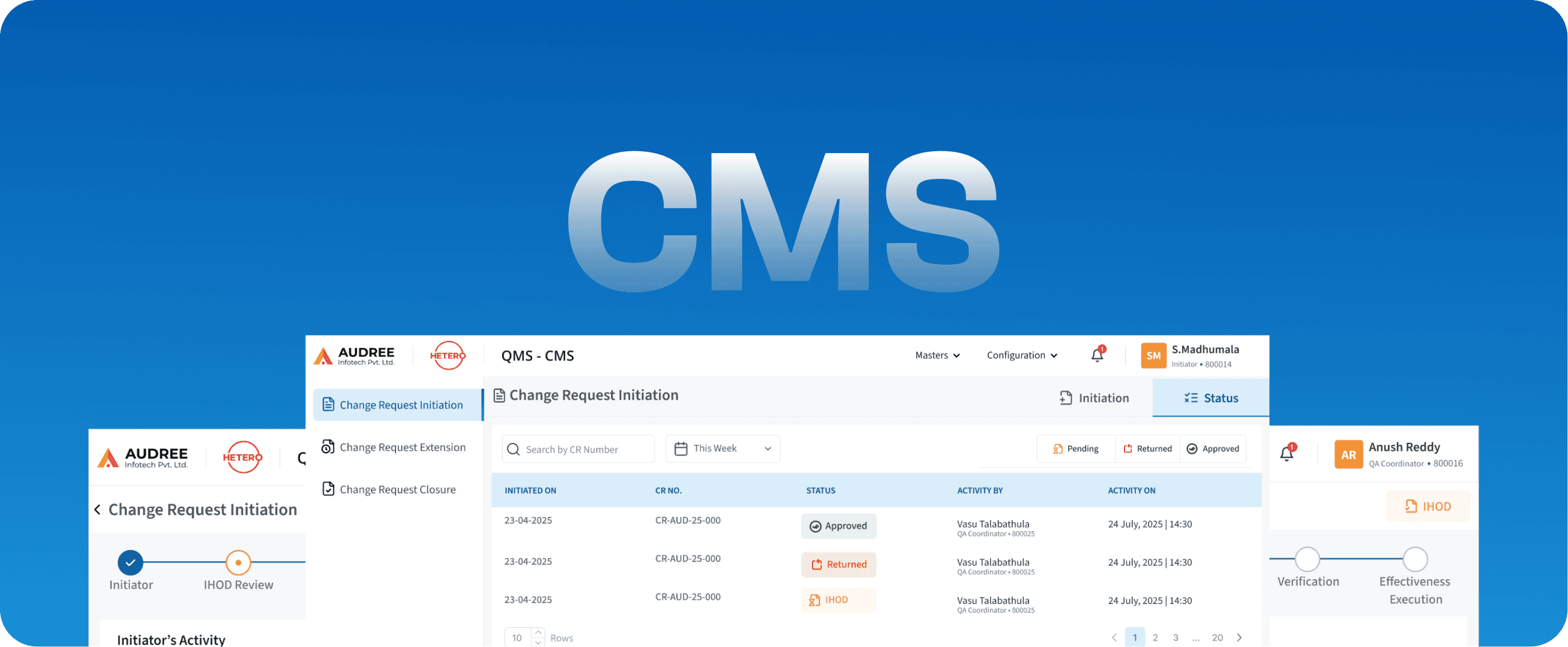
Change Management System
IMS
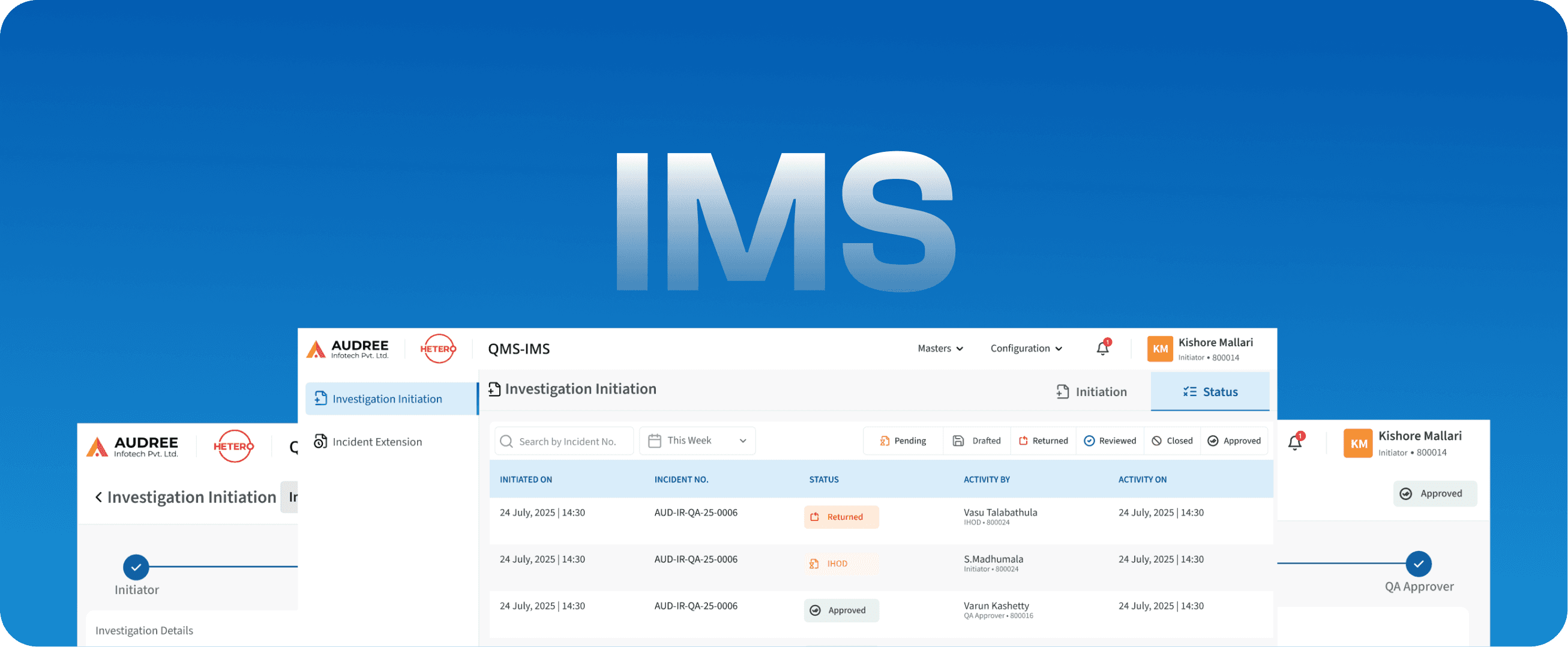
Incident Management System
BRMS-API
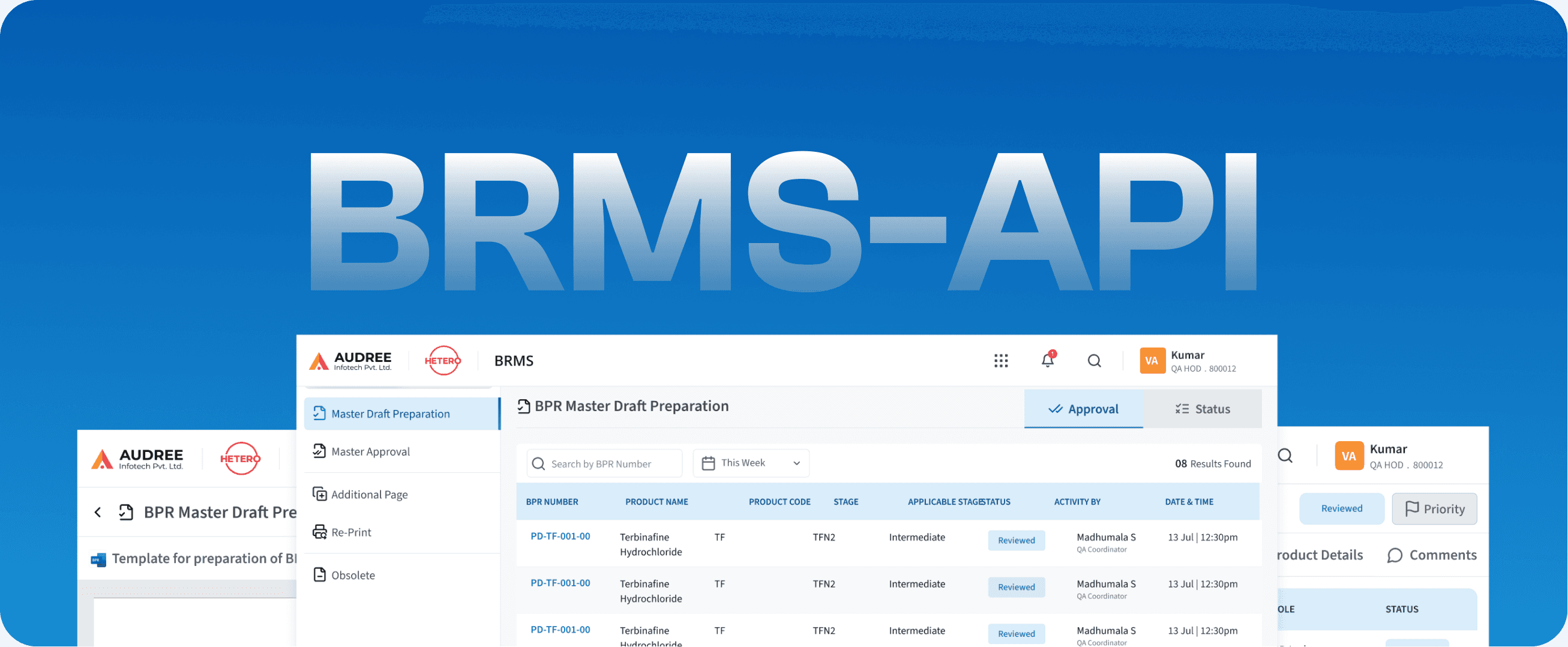
Batch Record- Active Pharmaceutical Ingredient
E-BRMS
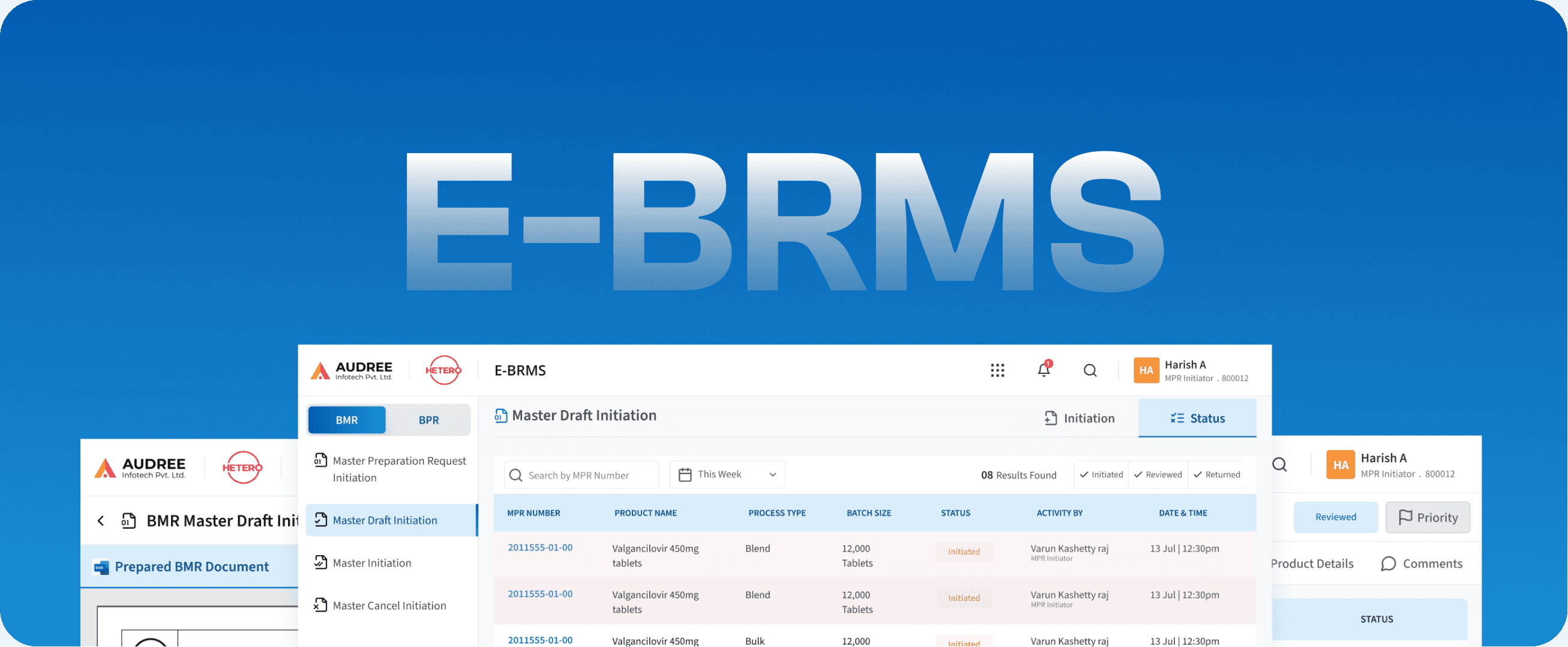
Batch Record Management System
RIMS
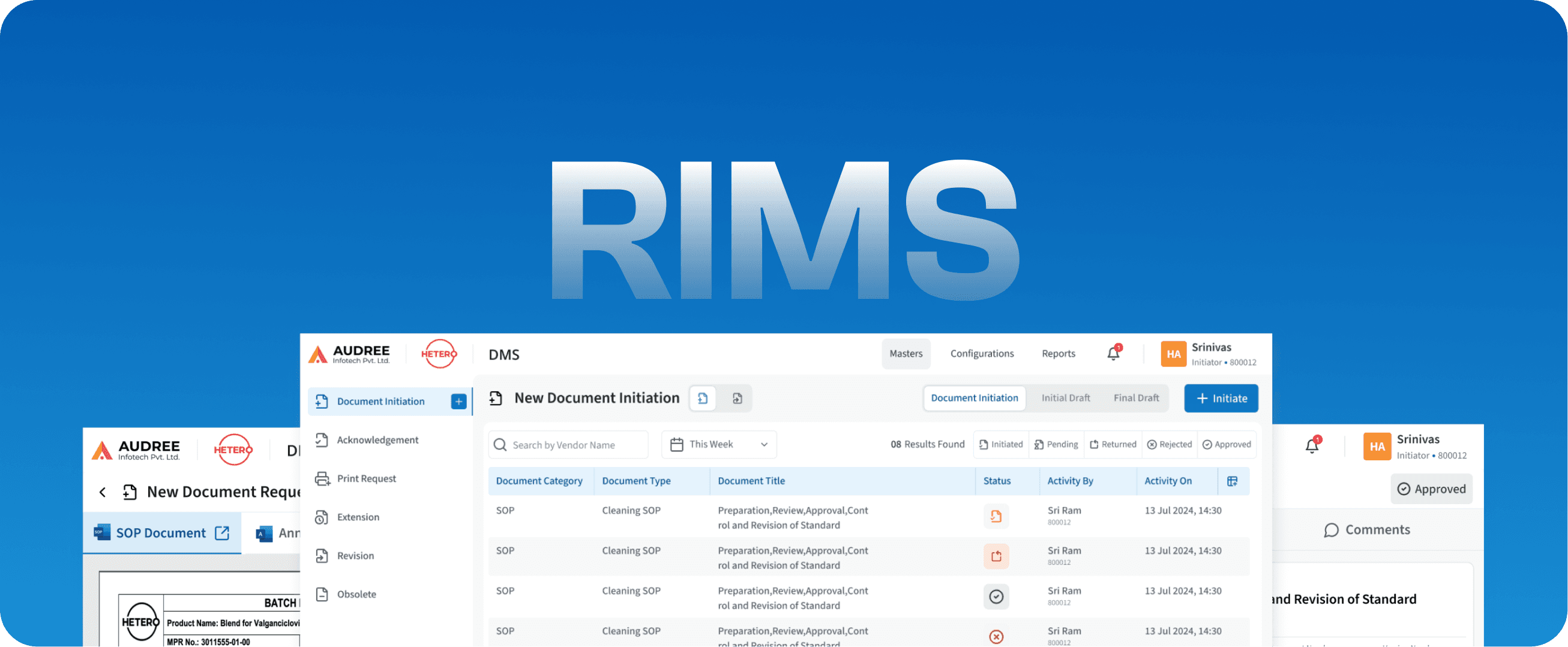
Regulatory Information Management System
APQR
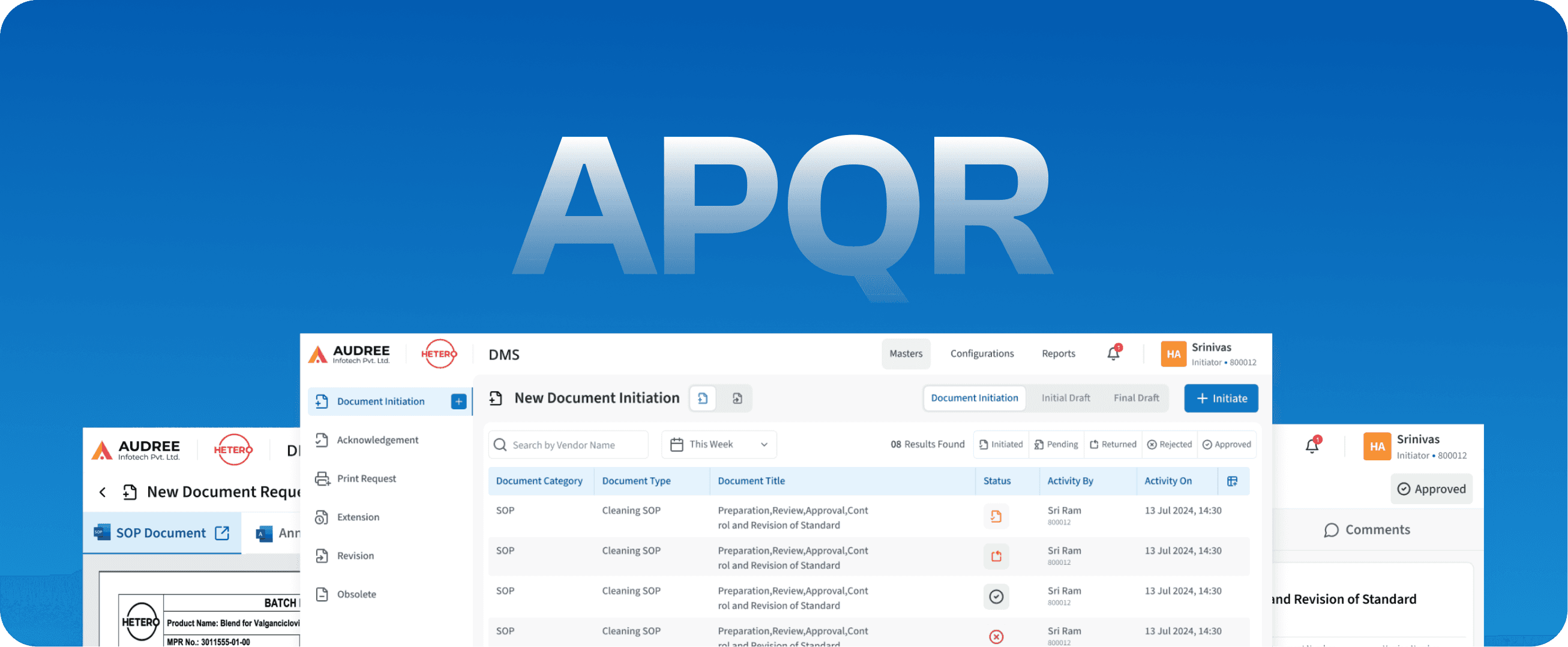
Annual Product Quality Review
RCAI

Root Cause Analysis with Intelligence
LMS

Learning Management System
LIMS

Laboratory Information Management System
S & OP

Sales & Operations Planning
E-BMR

Batch Manufacturing Recall
RIMS
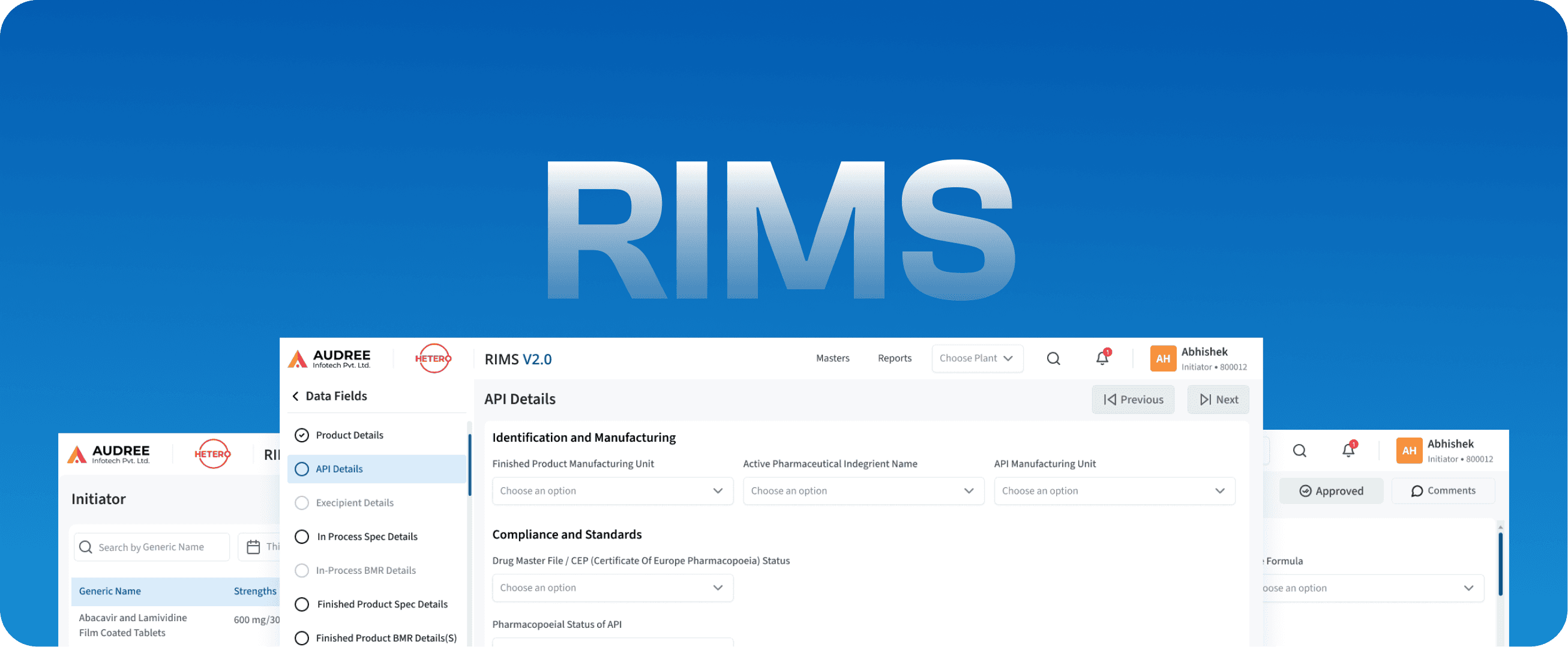
Regulatory Information Management Systems
IMS

Incident Management System
BRMS-API

Batch Record- Active Pharmaceutical Ingredient
E-BRMS

Batch Record Management System
APQR

Annual Product Quality Review
RIMS

Regulatory Information Management System
WMPS

Warehouse Management System
DMS

Document Management System
CMS

Change Management System
OOS

Out Of Specification
LIR-AER

Laboratory Information Record
Vendor Portal

Vendor Management System
QAS

Quality Agreement System
CAPA

Corrective And Preventive Actions
Impact
Impact
Impact
Before the redesign,
Before the redesign,
Before the redesign,
adoption was limited to 4 plants in 2 countries.
adoption was limited
adoption was
limited to 4 plants in 2 countries.
to 4 plants in 2 countries.
After the redesign the applications are actively used by
After the redesign
After the redesign the applications
are actively used by
the applications are actively used by
35+ Pharma Facilities
35+ Pharma Facilities
Spread Across
Spread Across
6+ countries Around the Globe
6+ countries Around the Globe
35+ Pharma Facilities
Spread Across
6+ countries Around the Globe













Make your product the one users recommend!
Make your product the one users recommend!
Make your product the one users recommend!