Redesigning LIMS to Simplify End-to-End Laboratory Workflows
Redesigning LIMS to Simplify End-to-End Laboratory Workflows
Redesigning LIMS to Simplify End-to-End Laboratory Workflows
About Project
About Project
About Project
The Laboratory Information Management System (LIMS) is the backbone of Pharma laboratory operations, used to manage samples, tests, results, approvals, and compliance documentation across QC and QA teams.
Audree was previously using an external LIMS (Caliber), but its highly modular, complex structure and fragmented workflows made day-to-day lab operations difficult to navigate and heavily dependent on training.
The system’s complexity led to confusion, manual workarounds, and inefficiencies during routine laboratory tasks. To overcome these challenges, Audree decided to build its own LIMS redesigning the experience into a clear, guided, and compliance-ready digital workflow aligned with real laboratory practices. The goal was to reduce manual effort, simplify execution, and enable audit-ready operations at scale.
The Laboratory Information Management System (LIMS) is the backbone of Pharma laboratory operations, used to manage samples, tests, results, approvals, and compliance documentation across QC and QA teams.
Audree was previously using an external LIMS (Caliber), but its highly modular, complex structure and fragmented workflows made day-to-day lab operations difficult to navigate and heavily dependent on training.
The system’s complexity led to confusion, manual workarounds, and inefficiencies during routine laboratory tasks. To overcome these challenges, Audree decided to build its own LIMS redesigning the experience into a clear, guided, and compliance-ready digital workflow aligned with real laboratory practices. The goal was to reduce manual effort, simplify execution, and enable audit-ready operations at scale.
About Project
Pharmaceutical
Team
Anush Reddy, S.Madhumala
Subscription Category
Quick win
Project start Year
August 2025
Core Business Challenges
Core Business Challenges
Core Business Challenges
Poor User Adoption Due to Outdated Experience
Poor User Adoption Due to Outdated Experience
Poor User Adoption Due to Outdated Experience
Overloaded screens and inconsistent workflows made LIMS hard to use. Poor navigation and low traceability made difficult to complete lab tasks.
Overloaded screens and inconsistent workflows made LIMS hard to use. Poor navigation and low traceability made difficult to complete lab tasks.
Overloaded screens and inconsistent workflows made LIMS hard to use. Poor navigation and low traceability made difficult to complete lab tasks.
Slowed by Process Inefficiency
Slowed by Process Inefficiency
Slowed by Process Inefficiency
Sample handling, testing, and approvals were spread across multiple modules and manual steps, slowing routine laboratory operations.
Sample handling, testing, and approvals were spread across multiple modules and manual steps, slowing routine laboratory operations.
Sample handling, testing, and approvals were spread across multiple modules and manual steps, slowing routine laboratory operations.
Heavy Dependence on Manual Coordination
Heavy Dependence on Manual Coordination
Limited visibility into sample status, test progress existed but was hard to access, pushing teams to rely on emails, calls, and offline tracking
Limited visibility into sample status, test progress existed but was hard to access, pushing teams to rely on emails, calls, and offline tracking
Heavy Dependence on Manual Coordination
Limited visibility into sample status, test progress existed but was hard to access, pushing teams to rely on emails, calls, and offline tracking
Our Approach
Our Approach
Our Approach
Mapping User Flows to Uncover Hidden Gaps
We mapped the complete LIMS lifecycle from sample login and test execution to result review, approvals, and final closure.
This uncovered fragmented workflows, unclear role ownership, and limited visibility across QC analysts, reviewers, and QA teams.
Mapping User Flows to Uncover Hidden Gaps
We mapped the complete LIMS lifecycle from sample login and test execution to result review, approvals, and final closure.
This uncovered fragmented workflows, unclear role ownership, and limited visibility across QC analysts, reviewers, and QA teams.
Mapping User Flows to Uncover Hidden Gaps
We mapped the complete LIMS lifecycle from sample login and test execution to result review, approvals, and final closure.
This uncovered fragmented workflows, unclear role ownership, and limited visibility across QC analysts, reviewers, and QA teams.
Designing LIMS Into a Structured Laboratory Workflow
Designing LIMS Into a Structured Laboratory Workflow
Designing LIMS Into a Structured Laboratory Workflow
We redesigned Audree’s LIMS into a role-driven, guided laboratory workflow that resolved navigation challenges and improved visibility across modules. Clear sample lifecycles, contextual views, and controlled approvals replaced disconnected tracking enabling faster execution, better accuracy, and audit-ready sample, test, and result management.
We redesigned Audree’s LIMS into a role-driven, guided laboratory workflow that resolved navigation challenges and improved visibility across modules. Clear sample lifecycles, contextual views, and controlled approvals replaced disconnected tracking enabling faster execution, better accuracy, and audit-ready sample, test, and result management.
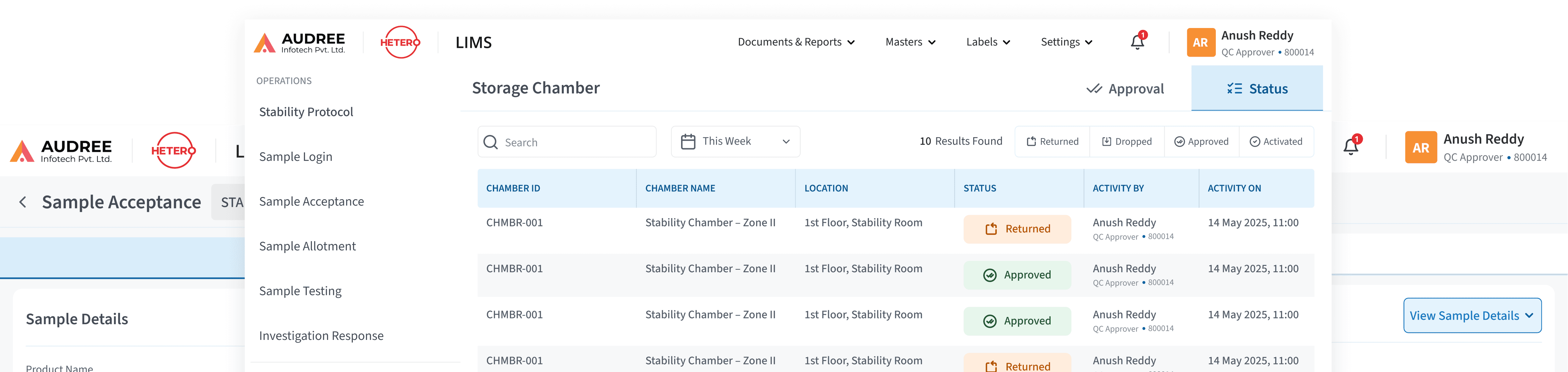




Guided Sample-to-Test Journeys
Guided Sample-to-Test Journeys
Guided Sample-to-Test Journeys
We transformed fragmented sample handling into a clear, step-by-step lifecycle from sample login to testing, review, and closure.
Each stage surfaces only the relevant actions, inputs, and context, ensuring users always know where the sample stands, what’s next, and who owns the action reducing confusion, manual checks, and execution errors for QC teams.
We transformed fragmented sample handling into a clear, step-by-step lifecycle from sample login to testing, review, and closure.
Each stage surfaces only the relevant actions, inputs, and context, ensuring users always know where the sample stands, what’s next, and who owns the action reducing confusion, manual checks, and execution errors for QC teams.
We transformed fragmented sample handling into a clear, step-by-step lifecycle from sample login to testing, review, and closure.
Each stage surfaces only the relevant actions, inputs, and context, ensuring users always know where the sample stands, what’s next, and who owns the action reducing confusion, manual checks, and execution errors for QC teams.
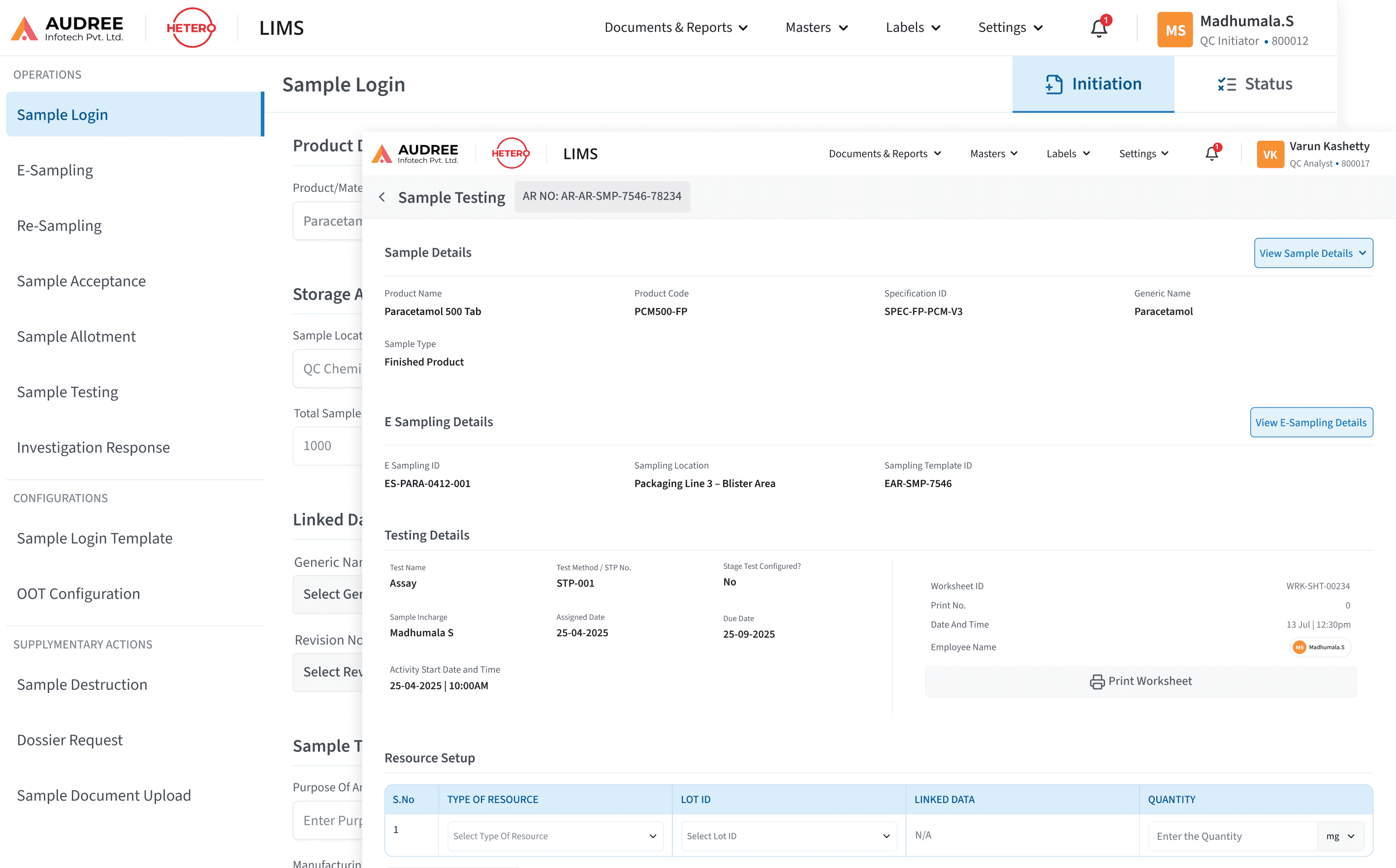
Data-First Interfaces for Faster Decisions
Data-First Interfaces for Faster Decisions
Data-First Interfaces for Faster Decisions
Test results, inventory usage, instrument status, and approvals are presented through clean tables, clear statuses, and contextual views eliminating cross-referencing between modules, spreadsheets, and documents, and enabling faster reviews, confident approvals, and less backtracking during investigations or audits.
Test results, inventory usage, instrument status, and approvals are presented through clean tables, clear statuses, and contextual views eliminating cross-referencing between modules, spreadsheets, and documents, and enabling faster reviews, confident approvals, and less backtracking during investigations or audits.
Test results, inventory usage, instrument status, and approvals are presented through clean tables, clear statuses, and contextual views eliminating cross-referencing between modules, spreadsheets, and documents, and enabling faster reviews, confident approvals, and less backtracking during investigations or audits.
Clear Demand & Procurement Views for Confident Planning
Clear Demand & Procurement Views for Confident Planning
Clear Demand & Procurement Views for Confident Planning
Dedicated views for Procurement and Demand present critical planning data in a structured, easy-to-scan format.
In the Demand view, a simple FDF / API toggle allows users to switch instantly between finished dosage and API demand. This makes it easier to analyse requirements, compare volumes, and plan accurately without jumping between screens or reports.
Dedicated views for Procurement and Demand present critical planning data in a structured, easy-to-scan format.
In the Demand view, a simple FDF / API toggle allows users to switch instantly between finished dosage and API demand. This makes it easier to analyse requirements, compare volumes, and plan accurately without jumping between screens or reports.
Dedicated views for Procurement and Demand present critical planning data in a structured, easy-to-scan format.
In the Demand view, a simple FDF / API toggle allows users to switch instantly between finished dosage and API demand. This makes it easier to analyse requirements, compare volumes, and plan accurately without jumping between screens or reports.

A Clear, Self-Guided UI for Lab Execution
A Clear, Self-Guided UI for Lab Execution
A Clear, Self-Guided UI for Lab Execution
Consistent layouts, clear statuses, and role-based actions guide QC and QA teams through samples, tests, and approvals reducing errors, rework, and training dependency while staying audit-ready.
Consistent layouts, clear statuses, and role-based actions guide QC and QA teams through samples, tests, and approvals reducing errors, rework, and training dependency while staying audit-ready.




Result That Redefined Laboratory Operations
Result That Redefined Laboratory Operations
Result That Redefined Laboratory Operations
Structured sample flows, role-based actions, and centralised traceability improved execution accuracy, reduced errors, and strengthened inspection readiness across laboratories.
Structured sample flows, role-based actions, and centralised traceability improved execution accuracy, reduced errors, and strengthened inspection readiness across laboratories.



Stronger Compliance & Traceability
Stronger Compliance & Traceability
Stronger Compliance & Traceability
Centralised sample records, test data, approvals, and audit trails ensured every laboratory action was traceable, verifiable, and inspection-ready eliminating compliance gaps and manual evidence collection.
Centralised sample records, test data, approvals, and audit trails ensured every laboratory action was traceable, verifiable, and inspection-ready eliminating compliance gaps and manual evidence collection.
Centralised sample records, test data, approvals, and audit trails ensured every laboratory action was traceable, verifiable, and inspection-ready eliminating compliance gaps and manual evidence collection.

Faster API Batch Execution
Clear sample lifecycles, test-wise progression, and role-based actions enabled QC and QA teams to process samples, review results, and approve outcomes faster without cross checking multiple modules.

Fewer Support Tickets
Self-explanatory screens, contextual data, and guided workflows reduced errors, training dependency, and follow-ups enabling lab teams to work confidently with minimal support.


Faster API Batch Execution
Faster API Batch Execution
Clear sample lifecycles, test-wise progression, and role-based actions enabled QC and QA teams to process samples, review results, and approve outcomes faster without cross checking multiple modules.
Clear sample lifecycles, test-wise progression, and role-based actions enabled QC and QA teams to process samples, review results, and approve outcomes faster without cross checking multiple modules.


Fewer Support Tickets
Fewer Support Tickets
Self-explanatory screens, contextual data, and guided workflows reduced errors, training dependency, and follow-ups enabling lab teams to work confidently with minimal support.
Self-explanatory screens, contextual data, and guided workflows reduced errors, training dependency, and follow-ups enabling lab teams to work confidently with minimal support.
Deep-Dive Into More System
Deep-Dive Into More System
Deep-Dive Into More System
Browse every optimised Software and explore how legacy systems became intuitive.
Browse every optimised Software and explore how legacy systems became intuitive.
Browse every optimised Software and explore how legacy systems became intuitive.
RCAI

Root Cause Analysis with Intelligence
LMS
Learning Management System
LIMS
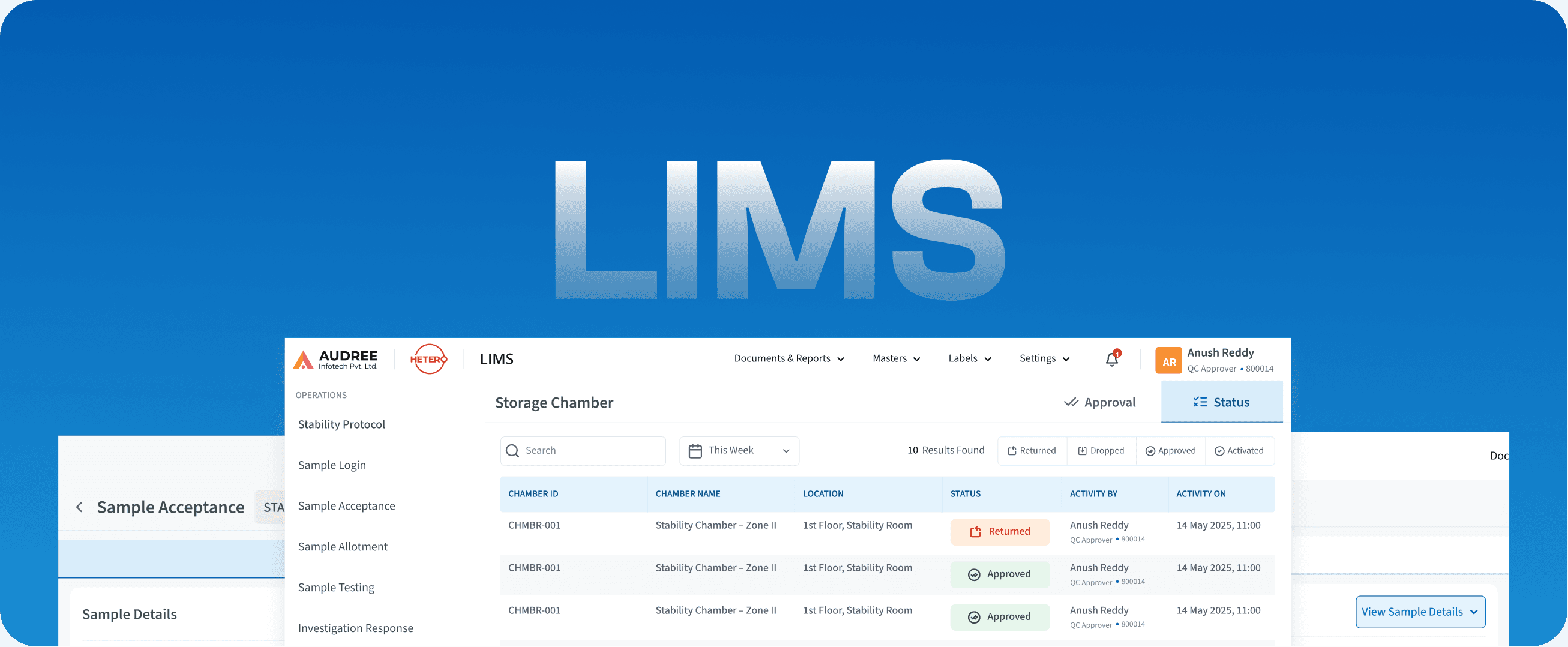
Laboratory Information Management System
S & OP
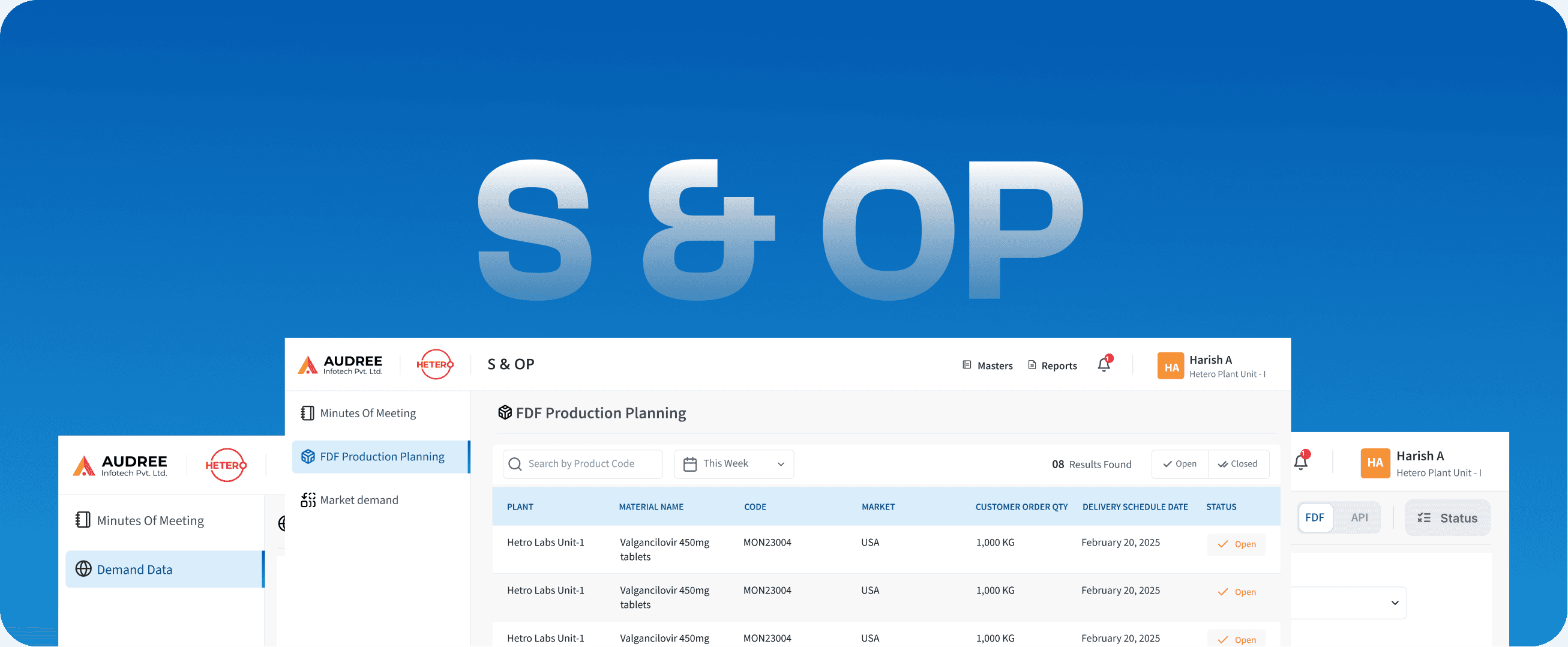
Sales & Operations Planning
E-BMR
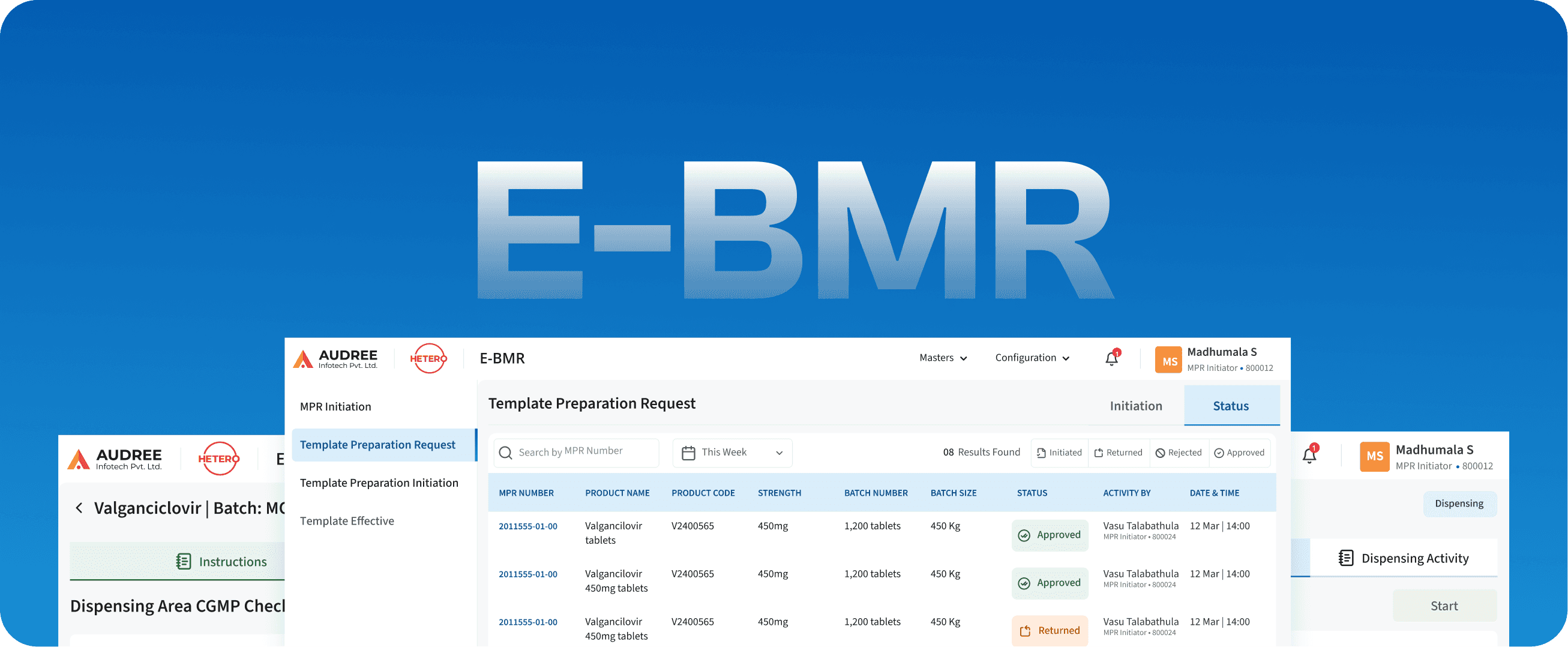
Batch Manufacturing Recall
WMPS

Warehouse Management System
DMS
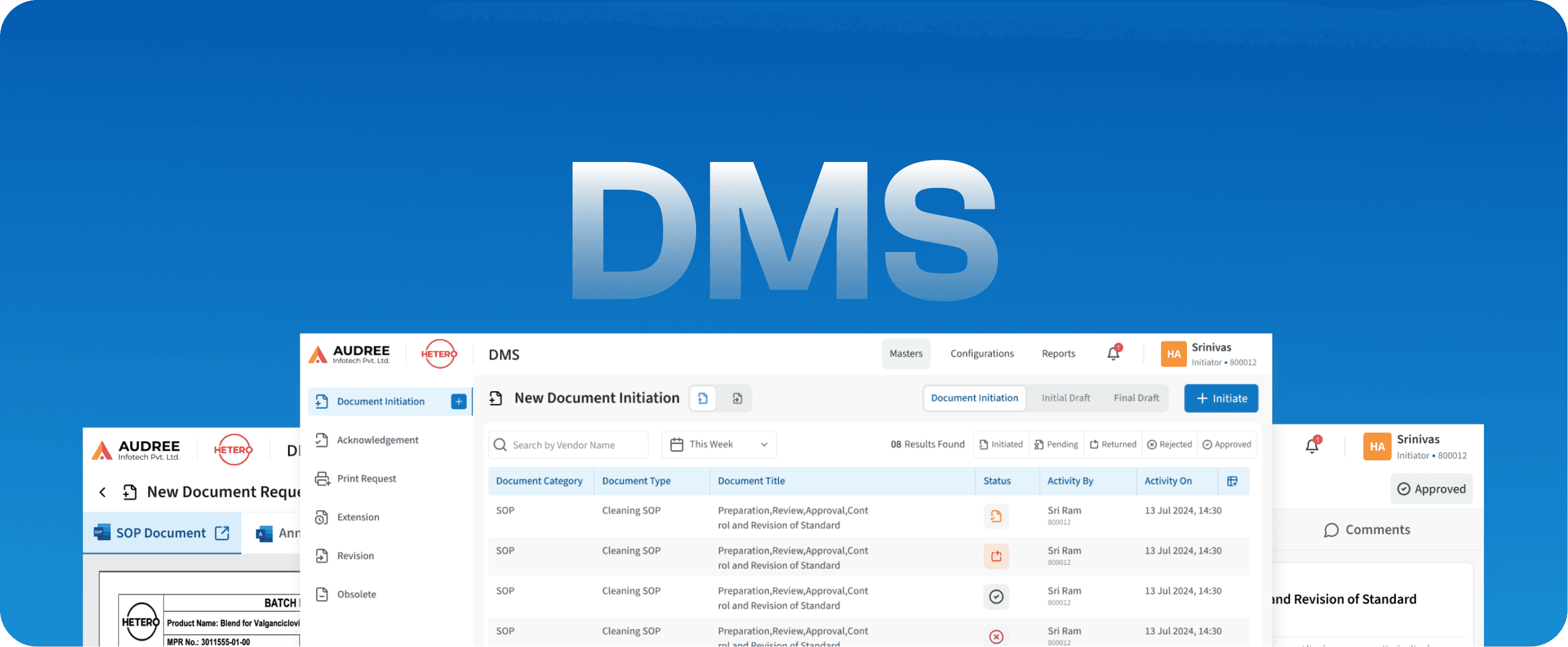
Document Management System
CAPA

Corrective And Preventive Actions
QAS

Quality Agreement System
Vendor Portal
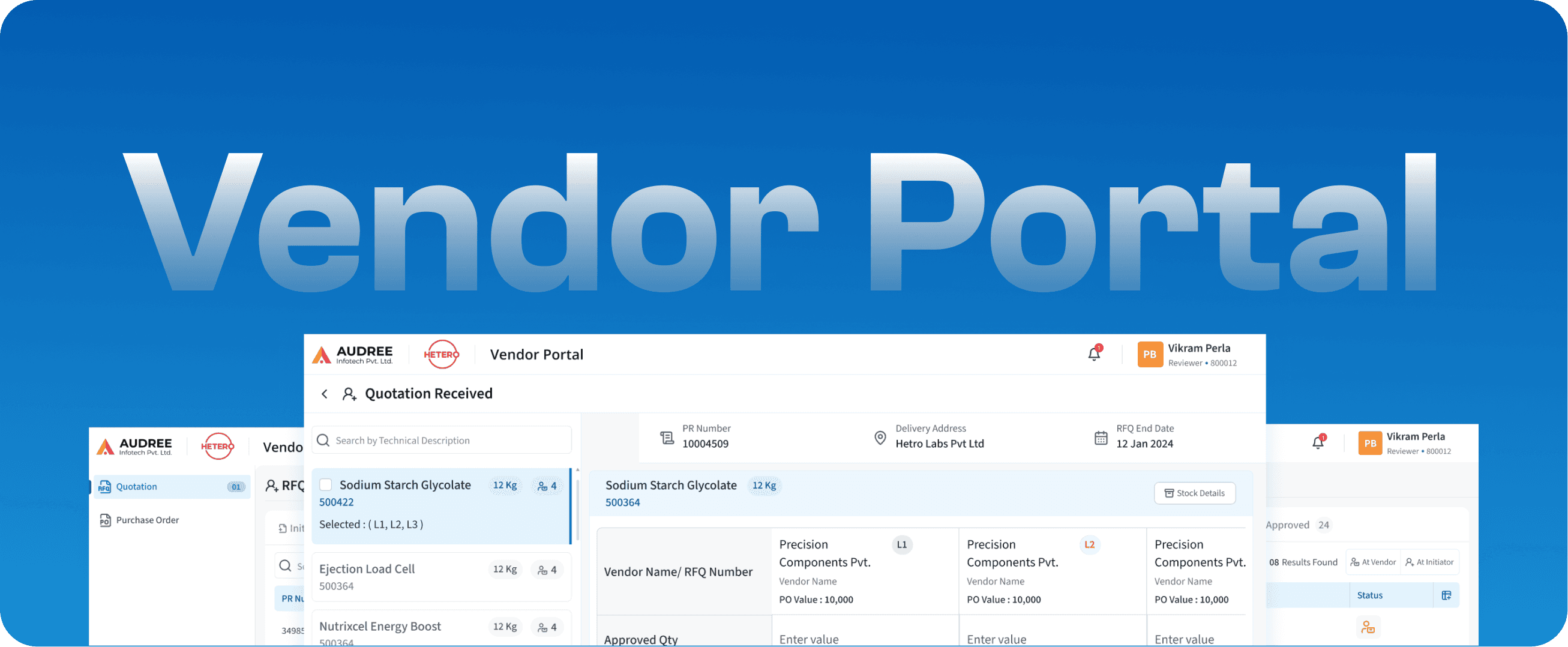
Vendor Management System
LIR-AER
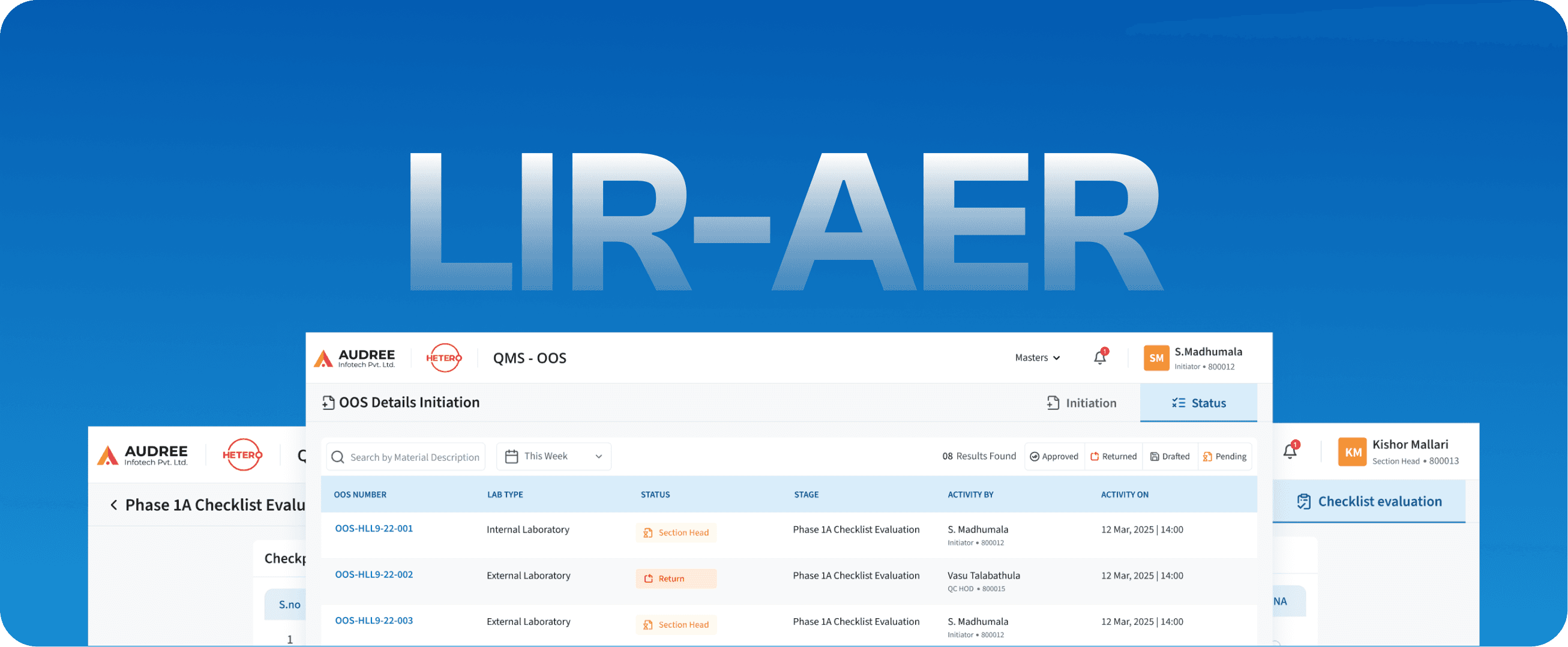
Laboratory Information Record
OOS
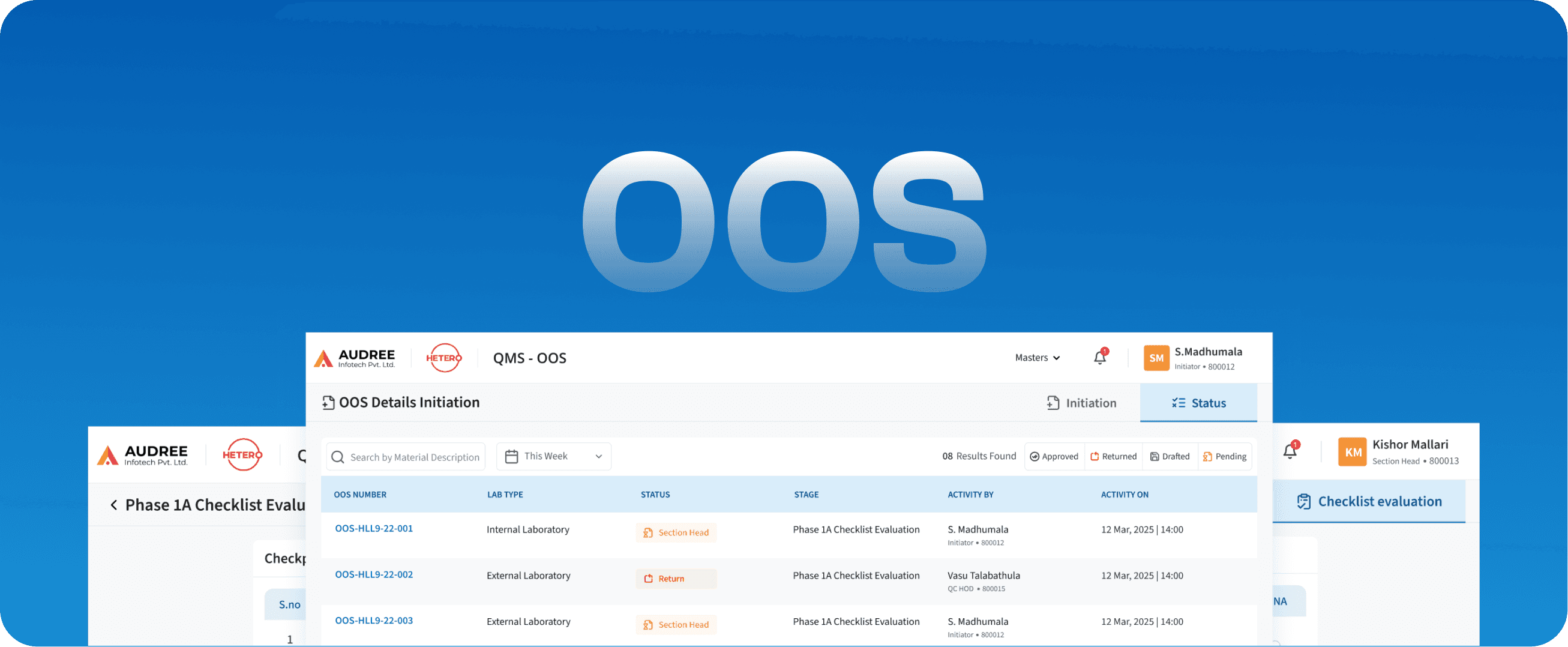
Out Of Specification
CMS
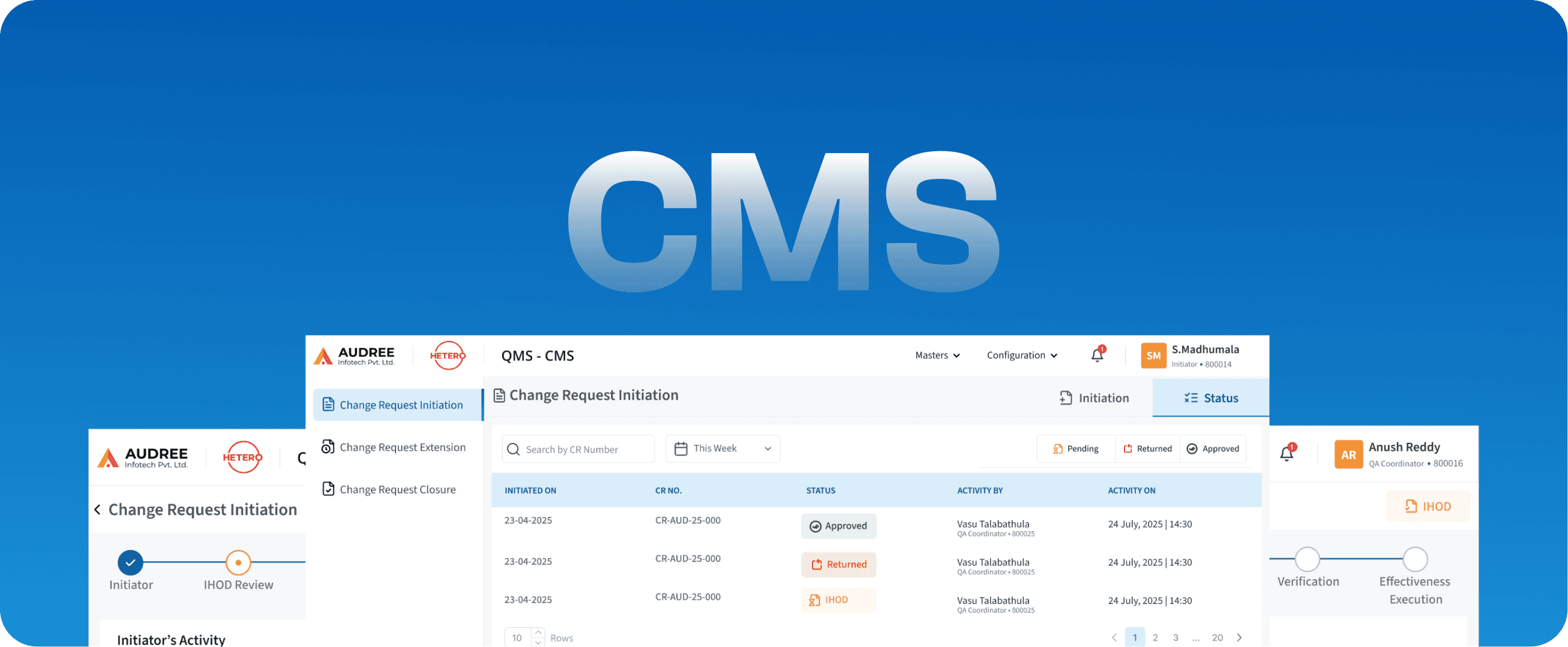
Change Management System
IMS
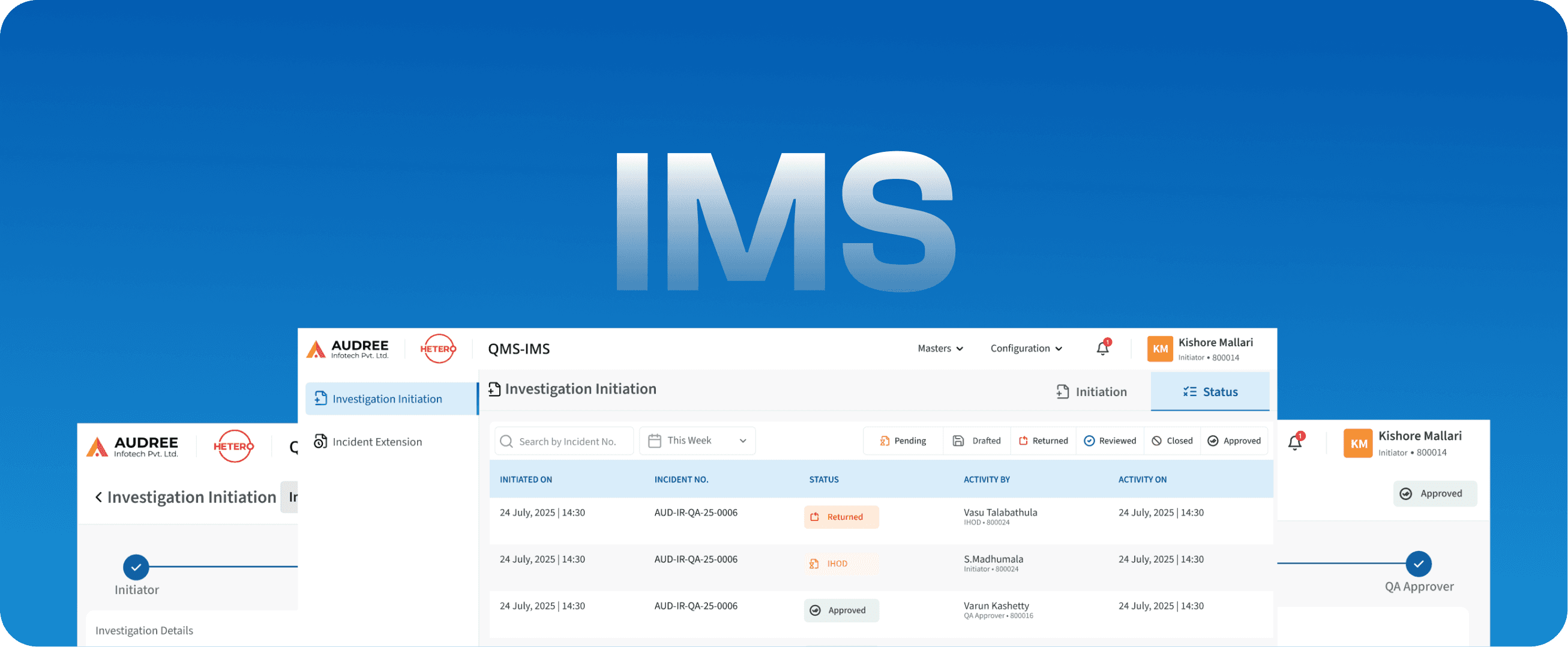
Incident Management System
BRMS-API
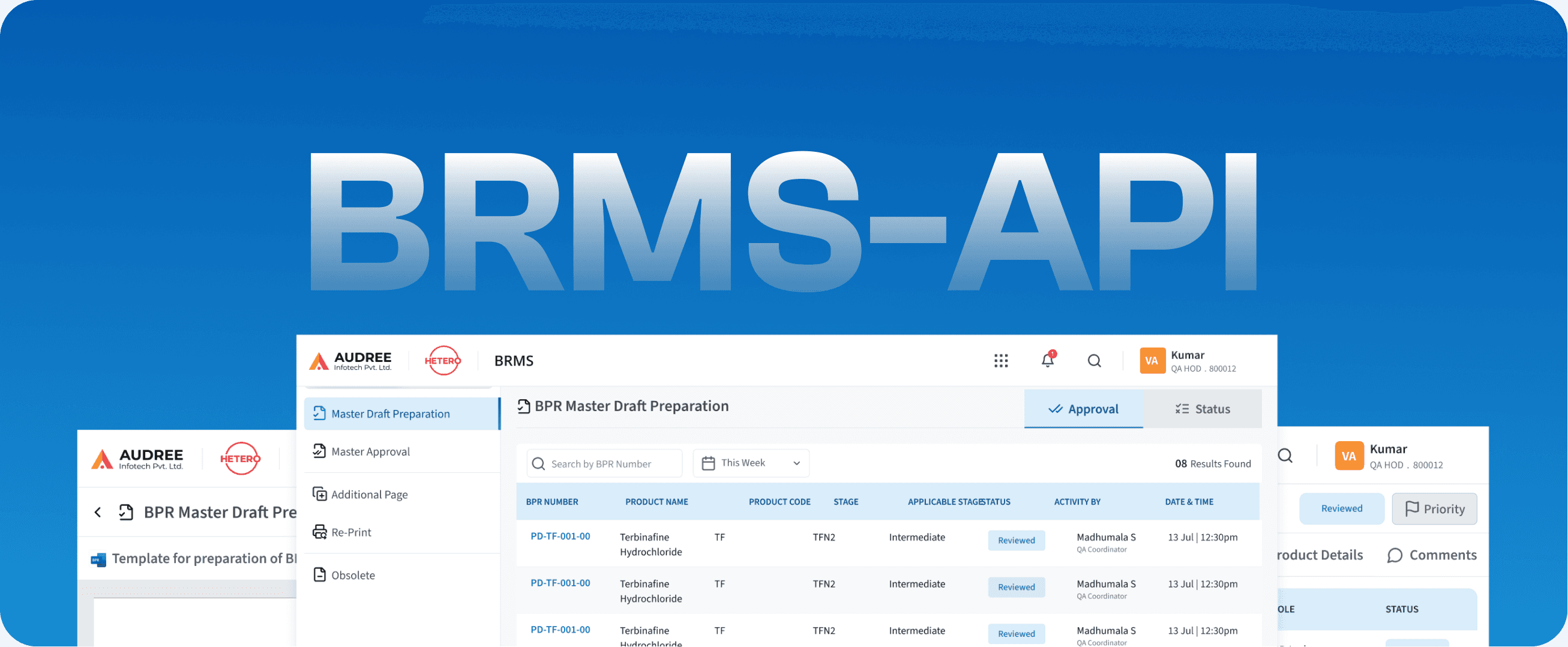
Batch Record- Active Pharmaceutical Ingredient
E-BRMS
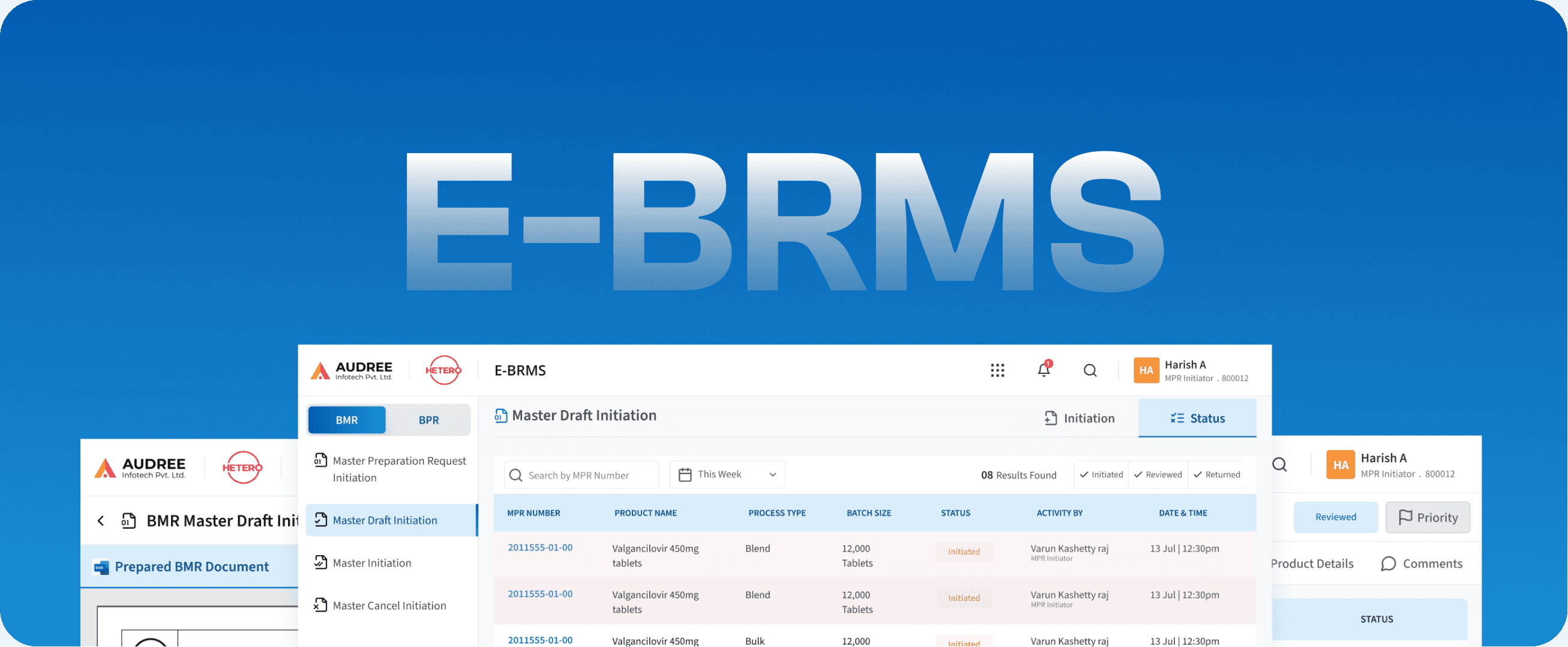
Batch Record Management System
RIMS
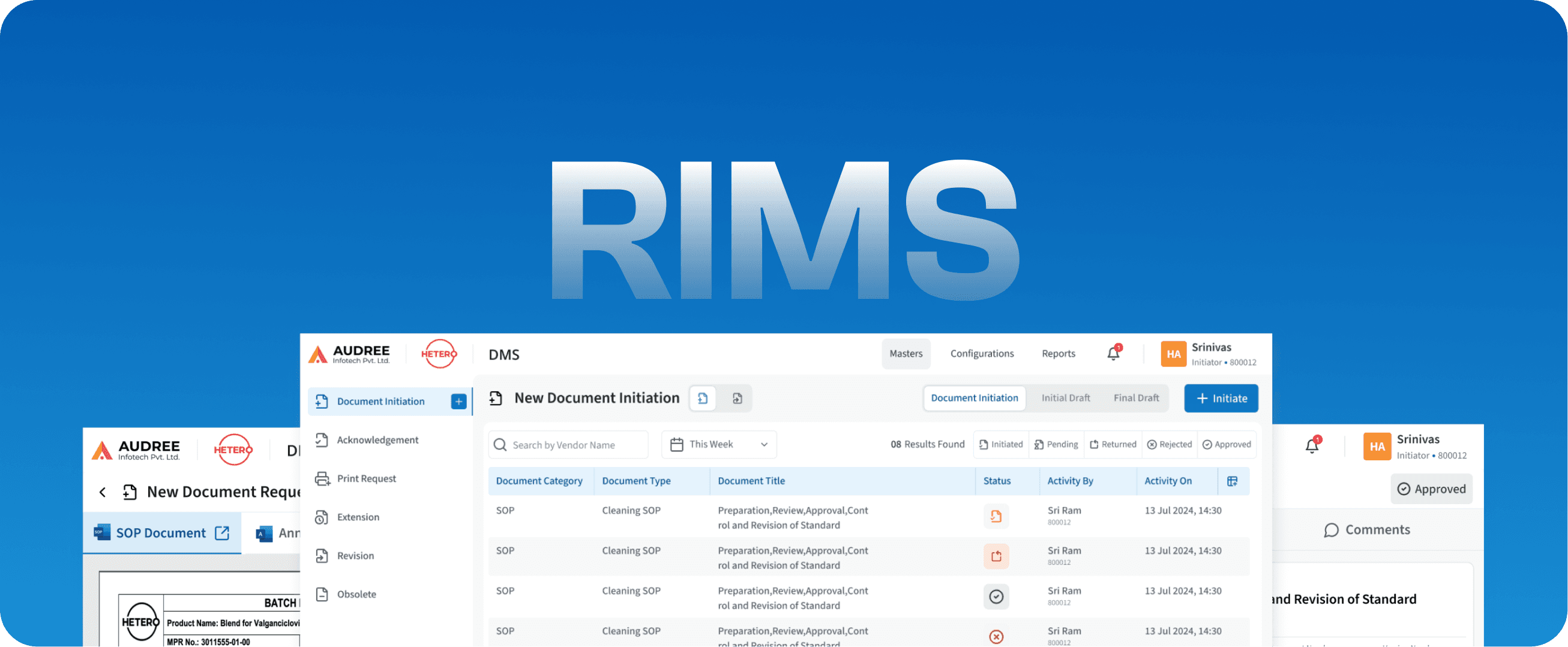
Regulatory Information Management System
APQR
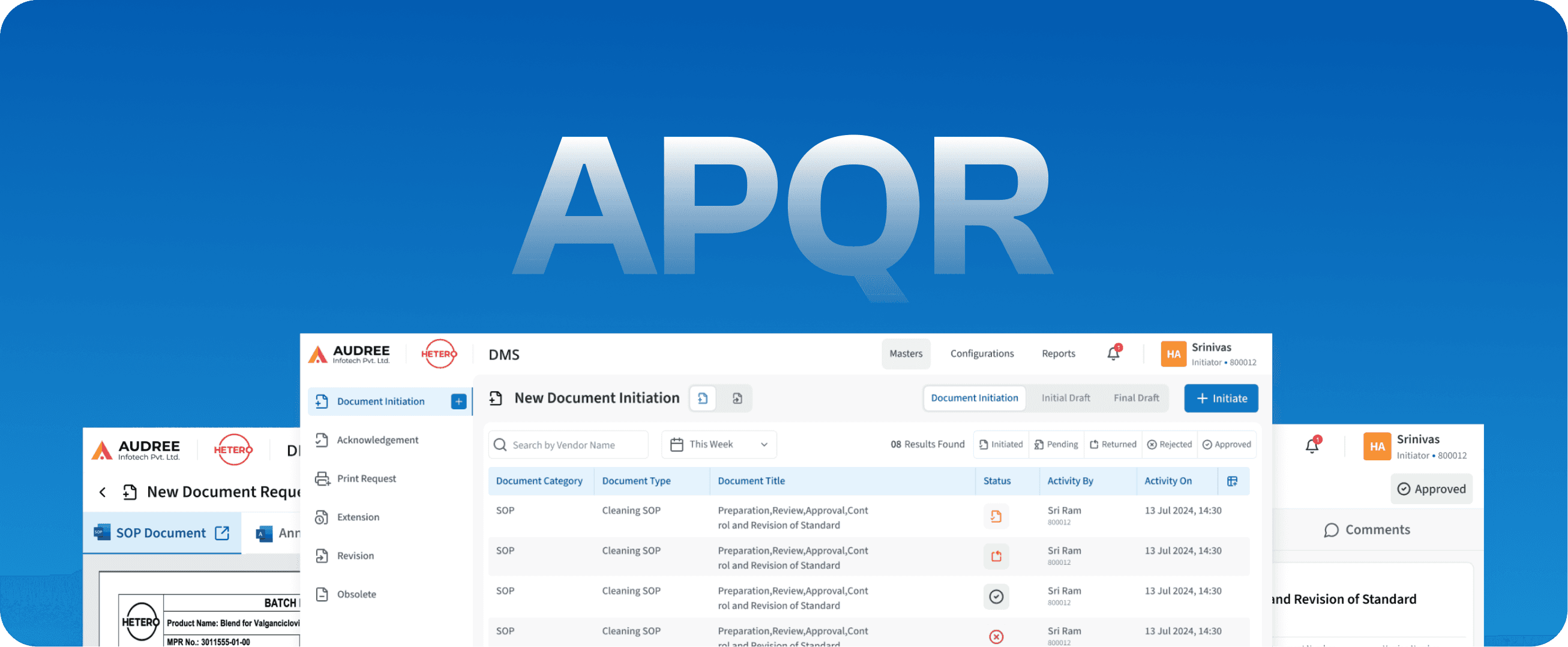
Annual Product Quality Review
RCAI

Root Cause Analysis with Intelligence
LMS

Learning Management System
LIMS

Laboratory Information Management System
S & OP

Sales & Operations Planning
E-BMR

Batch Manufacturing Recall
RIMS
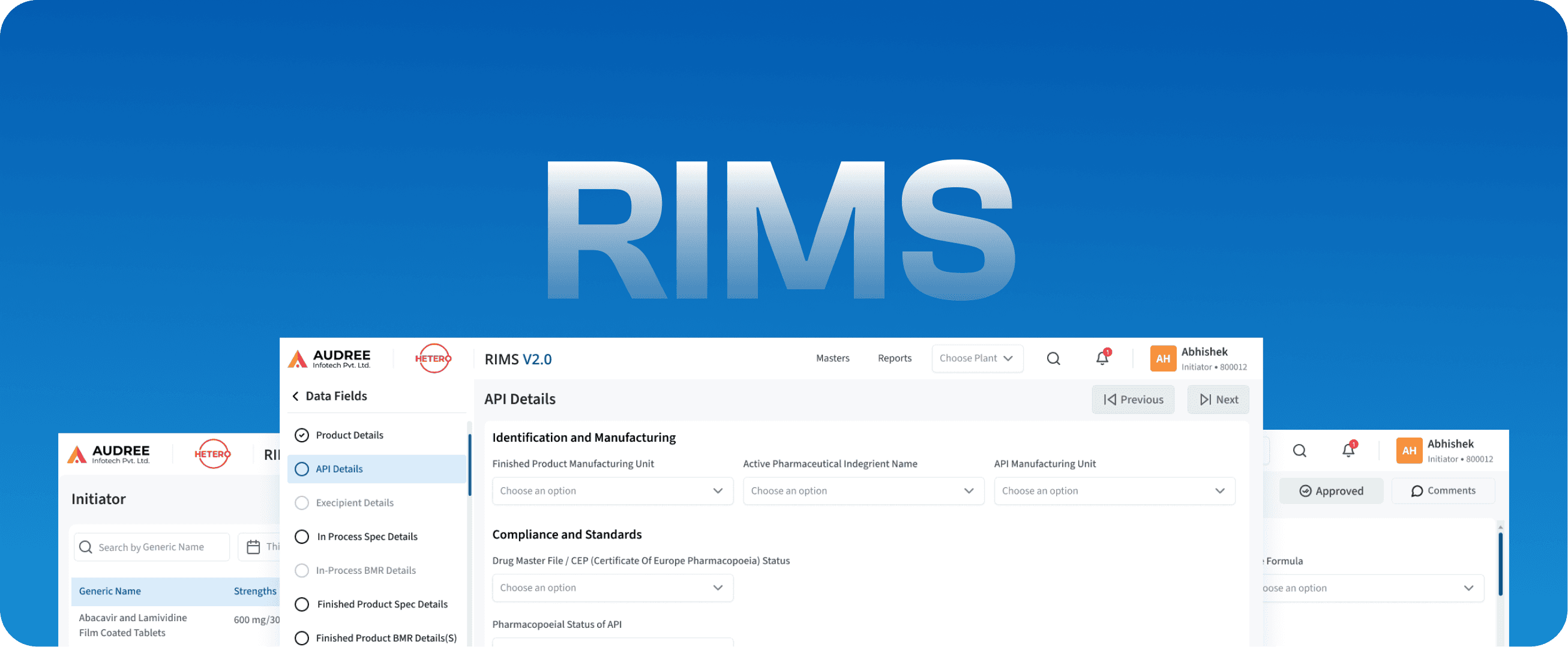
Regulatory Information Management Systems
IMS

Incident Management System
BRMS-API

Batch Record- Active Pharmaceutical Ingredient
E-BRMS

Batch Record Management System
APQR

Annual Product Quality Review
RIMS

Regulatory Information Management System
WMPS

Warehouse Management System
DMS

Document Management System
CMS

Change Management System
OOS

Out Of Specification
LIR-AER

Laboratory Information Record
Vendor Portal

Vendor Management System
QAS

Quality Agreement System
CAPA

Corrective And Preventive Actions

