Redesigning RIMS Into a Clear, Controlled Regulatory Workflow
Redesigning RIMS Into a Clear, Controlled Regulatory Workflow
Redesigning RIMS Into a Clear, Controlled Regulatory Workflow
About Project
About Project
About Project
Regulatory Information Management Systems (RIMS) help Regulatory Affairs teams manage product registrations, submissions, variations, renewals, and country specific regulatory data across the product lifecycle.
Regulatory teams struggled with fragmented data, poor visibility into submission status and timelines, and reliance on offline trackers, making coordination across products, countries, and submissions inefficient and error prone.
To resolve these challenges, Audree approached us to redesign their RIMS. We transformed the end-to-end regulatory lifecycle into a structured, role aligned digital workflow that reflects how regulatory teams plan, execute, and track submissions in real-world environments.
Regulatory Information Management Systems (RIMS) help Regulatory Affairs teams manage product registrations, submissions, variations, renewals, and country specific regulatory data across the product lifecycle.
Regulatory teams struggled with fragmented data, poor visibility into submission status and timelines, and reliance on offline trackers, making coordination across products, countries, and submissions inefficient and error prone.
To resolve these challenges, Audree approached us to redesign their RIMS. We transformed the end-to-end regulatory lifecycle into a structured, role aligned digital workflow that reflects how regulatory teams plan, execute, and track submissions in real-world environments.
About Project
Pharmaceutical
Team
Abhishek Jha, T.Srinivas
Subscription Category
Quick win
Project start Year
August 2024
Core Business Challenges
Core Business Challenges
Core Business Challenges
Poor User Adoption Due to Outdated Experience
Poor User Adoption Due to Outdated Experience
Poor User Adoption Due to Outdated Experience
An outdated interface and unclear lifecycle forced teams to cross check data just to track submission status.
An outdated interface and unclear lifecycle forced teams to cross check data just to track submission status.
An outdated interface and unclear lifecycle forced teams to cross check data just to track submission status.
Slowed by Fragmented Regulatory Processes
Slowed by Fragmented Regulatory Processes
Slowed by Fragmented Regulatory Processes
Scattered data, repeated entries, and unclear submission flows slowed regulatory work that demands speed, accuracy, and compliance.
Scattered data, repeated entries, and unclear submission flows slowed regulatory work that demands speed, accuracy, and compliance.
Scattered data, repeated entries, and unclear submission flows slowed regulatory work that demands speed, accuracy, and compliance.
Heavy Dependence on Manual Coordination
Heavy Dependence on Manual Coordination
Teams relied on emails, calls, and spreadsheets to validate submissions, slowing reviews and increasing QA workload.
Teams relied on emails, calls, and spreadsheets to validate submissions, slowing reviews and increasing QA workload.
Heavy Dependence on Manual Coordination
Teams relied on emails, calls, and spreadsheets to validate submissions, slowing reviews and increasing QA workload.
Our Approach
Our Approach
Our Approach
Mapping User Flows to Uncover Hidden Gaps
We mapped the entire RIMS lifecycle from product and country setup to submission, review, and post-approval updates to uncover where teams faced fragmented data, unclear handoffs, and gaps in submission tracking across regions and stages.
Mapping User Flows to Uncover Hidden Gaps
We mapped the entire RIMS lifecycle from product and country setup to submission, review, and post-approval updates to uncover where teams faced fragmented data, unclear handoffs, and gaps in submission tracking across regions and stages.
Mapping User Flows to Uncover Hidden Gaps
We mapped the entire RIMS lifecycle from product and country setup to submission, review, and post-approval updates to uncover where teams faced fragmented data, unclear handoffs, and gaps in submission tracking across regions and stages.
Designing Regulatory Management Into a Guided Workflow
Designing Regulatory Management Into a Guided Workflow
Designing Regulatory Management Into a Guided Workflow
We transformed fragmented regulatory activities into a single, structured workflow. Each stage from product and country setup to submission tracking was redesigned into clear, role-based steps with consistent data and improved visibility, reducing reliance on offline trackers and manual follow-ups.
We transformed fragmented regulatory activities into a single, structured workflow. Each stage from product and country setup to submission tracking was redesigned into clear, role-based steps with consistent data and improved visibility, reducing reliance on offline trackers and manual follow-ups.






Unified Handling of Europe and Non-Europe Transactions
Unified Handling of Europe and Non-Europe Transactions
Unified Handling of Europe and Non-Europe Transactions
We structured RIMS to clearly differentiate Europe and non-Europe regulatory transactions within the same system.
Dedicated tab-based views allow easy switching between regions, while region-specific fields, statuses, and submission paths ensure teams capture the right information upfront, track progress accurately, and manage variations without confusion while maintaining consistency across global regulatory operations.
We structured RIMS to clearly differentiate Europe and non-Europe regulatory transactions within the same system.
Dedicated tab-based views allow easy switching between regions, while region-specific fields, statuses, and submission paths ensure teams capture the right information upfront, track progress accurately, and manage variations without confusion while maintaining consistency across global regulatory operations.
We structured RIMS to clearly differentiate Europe and non-Europe regulatory transactions within the same system.
Dedicated tab-based views allow easy switching between regions, while region-specific fields, statuses, and submission paths ensure teams capture the right information upfront, track progress accurately, and manage variations without confusion while maintaining consistency across global regulatory operations.
Structured Phases for Seamless Investigation
Structured Phases for Seamless Investigation
Structured Phases for Seamless Investigation
We redesigned the RIMS data fields screen to bring clarity and structure to complex regulatory information. Related fields are grouped into logical sections such as product specifications, regulatory details, and manufacturing units, allowing users to enter and review data without jumping across screens.
We redesigned the RIMS data fields screen to bring clarity and structure to complex regulatory information. Related fields are grouped into logical sections such as product specifications, regulatory details, and manufacturing units, allowing users to enter and review data without jumping across screens.
We redesigned the RIMS data fields screen to bring clarity and structure to complex regulatory information. Related fields are grouped into logical sections such as product specifications, regulatory details, and manufacturing units, allowing users to enter and review data without jumping across screens.



Visual Cues That Simplify Regulatory Work
Visual Cues That Simplify Regulatory Work
Visual Cues That Simplify Regulatory Work
Status tags, submission insights, and structured stages turn complex regulatory work into clear, actionable steps. Teams instantly see progress and next actions.
Status tags, submission insights, and structured stages turn complex regulatory work into clear, actionable steps. Teams instantly see progress and next actions.








Structured UI for Clear Regulatory Work
Structured UI for Clear Regulatory Work
Structured UI for Clear Regulatory Work
Clean layouts, structured tables, and clear visual cues help teams track submissions confidently, reduce errors, and stay aligned across products, countries, and timelines.
Clean layouts, structured tables, and clear visual cues help teams track submissions confidently, reduce errors, and stay aligned across products, countries, and timelines.




Results That Transformed the Regulatory Lifecycle
Results That Transformed the Regulatory Lifecycle
Results That Transformed the Regulatory Lifecycle
Defined regulatory workflows with role-based actions and controlled reviews improved submission predictability, reduced rework, and strengthened audit readiness across regions and teams.
Defined regulatory workflows with role-based actions and controlled reviews improved submission predictability, reduced rework, and strengthened audit readiness across regions and teams.



Stronger Compliance & Traceability
Stronger Compliance & Traceability
Stronger Compliance & Traceability
Real-time visibility into submissions, approvals, and variations ensured accurate tracking of regulatory data, reduced compliance gaps, and improved audit preparedness across global markets.
Real-time visibility into submissions, approvals, and variations ensured accurate tracking of regulatory data, reduced compliance gaps, and improved audit preparedness across global markets.
Real-time visibility into submissions, approvals, and variations ensured accurate tracking of regulatory data, reduced compliance gaps, and improved audit preparedness across global markets.

Faster Submission & Approval Cycles
Structured workflows and clear status indicators replaced manual coordination, enabling faster reviews, smoother approvals, and consistent progress across regulatory stages.

Reduced Support Dependency
Unified views and clear submission statuses minimized confusion and manual follow-ups, lowering support tickets and improving overall system adoption among regulatory teams.


Faster Submission & Approval Cycles
Faster Submission & Approval Cycles
Structured workflows and clear status indicators replaced manual coordination, enabling faster reviews, smoother approvals, and consistent progress across regulatory stages.
Structured workflows and clear status indicators replaced manual coordination, enabling faster reviews, smoother approvals, and consistent progress across regulatory stages.


Reduced Support Dependency
Reduced Support Dependency
Unified views and clear submission statuses minimized confusion and manual follow-ups, lowering support tickets and improving overall system adoption among regulatory teams.
Unified views and clear submission statuses minimized confusion and manual follow-ups, lowering support tickets and improving overall system adoption among regulatory teams.
Deep-Dive Into More System
Deep-Dive Into More System
Deep-Dive Into More System
Browse every optimised Software and explore how legacy systems became intuitive.
Browse every optimised Software and explore how legacy systems became intuitive.
Browse every optimised Software and explore how legacy systems became intuitive.
RCAI

Root Cause Analysis with Intelligence
LMS
Learning Management System
LIMS
Laboratory Information Management System
S & OP
Sales & Operations Planning
E-BMR
Batch Manufacturing Recall
WMPS

Warehouse Management System
DMS
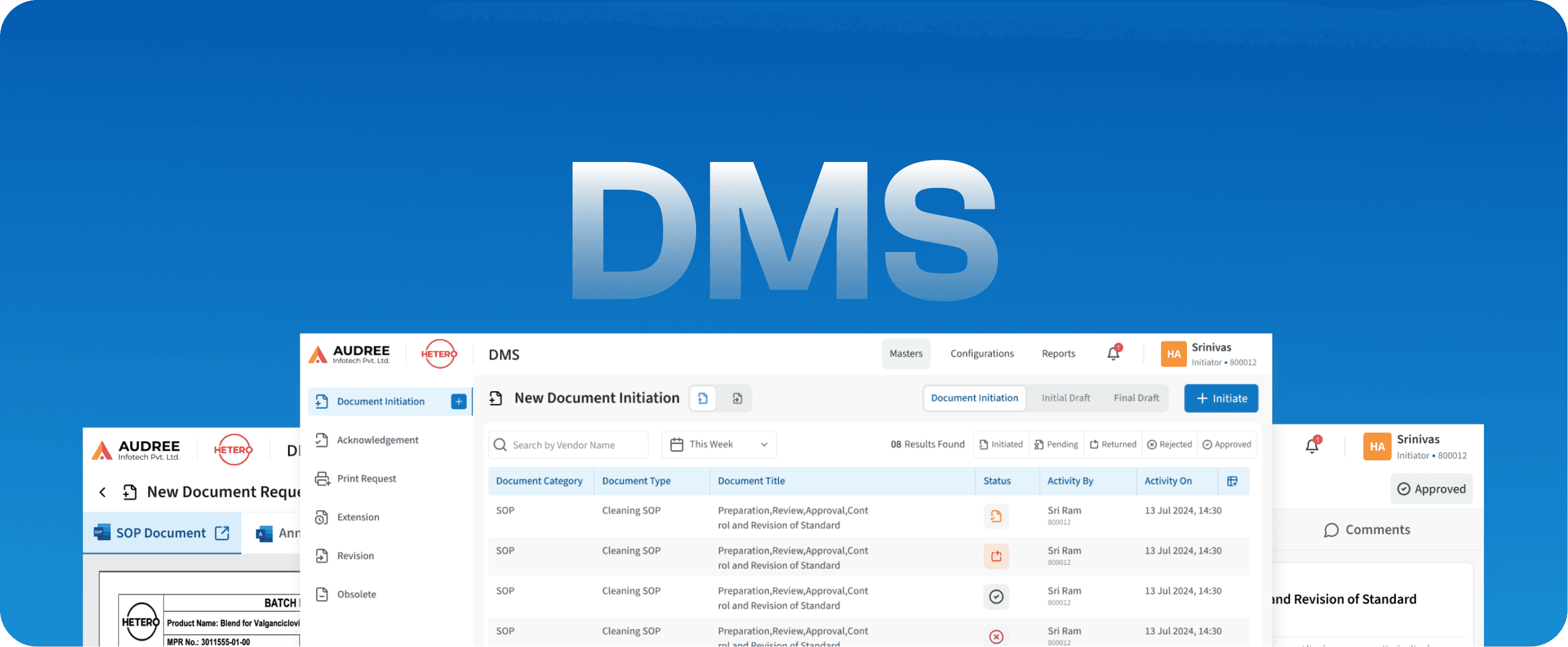
Document Management System
CAPA

Corrective And Preventive Actions
QAS

Quality Agreement System
Vendor Portal
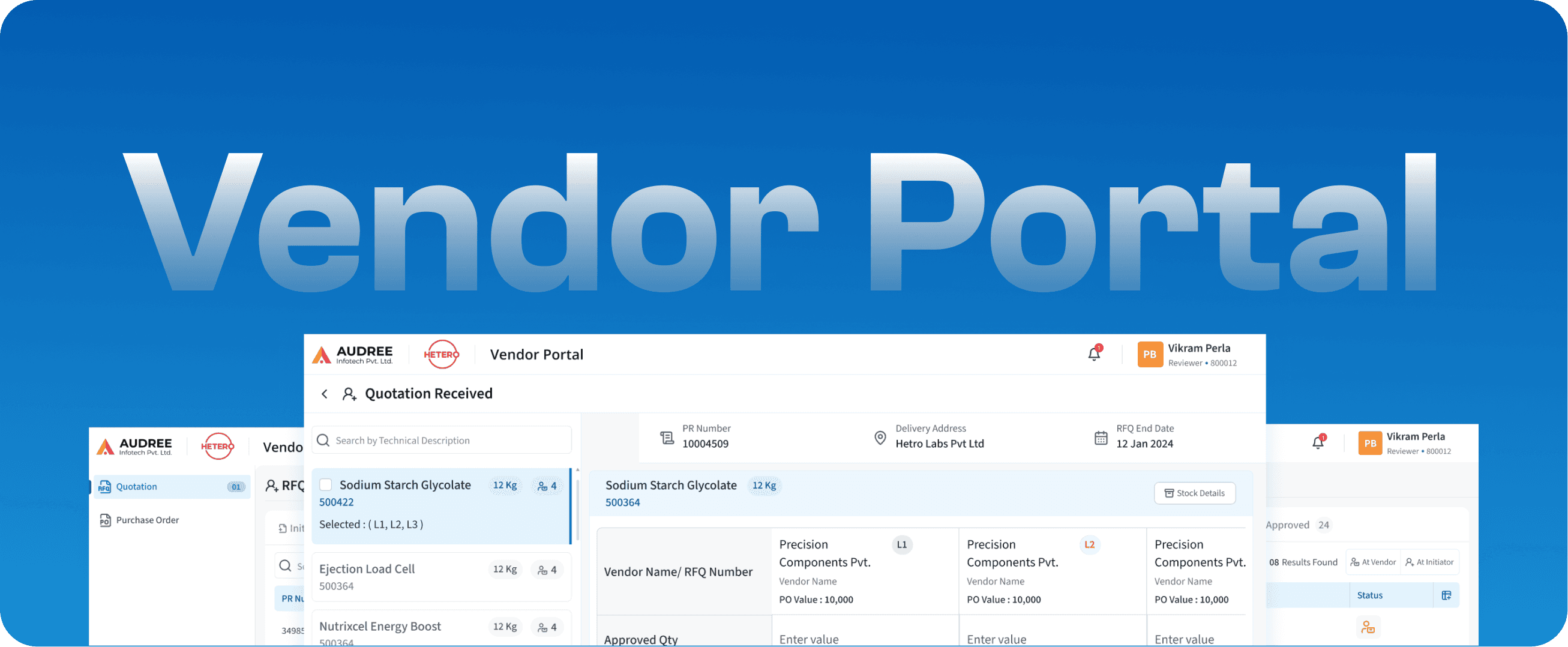
Vendor Management System
LIR-AER
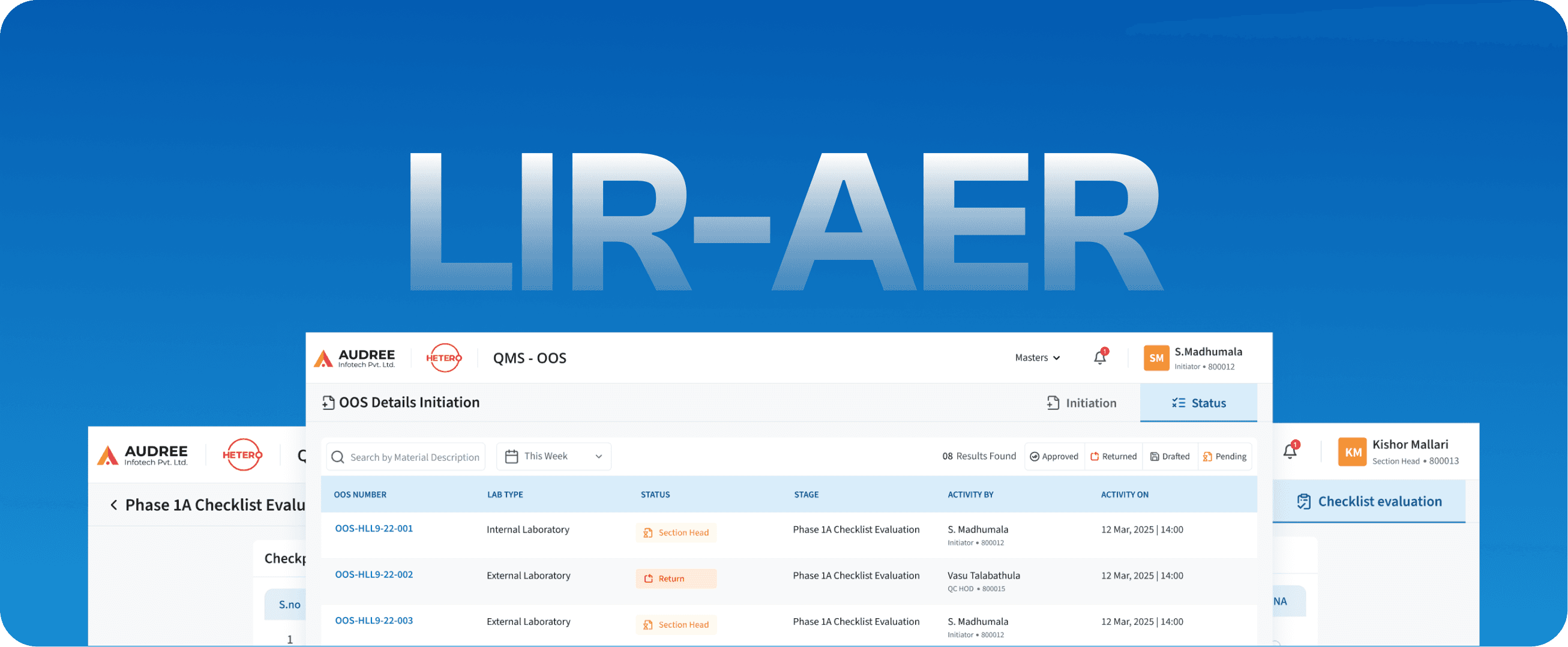
Laboratory Information Record
OOS
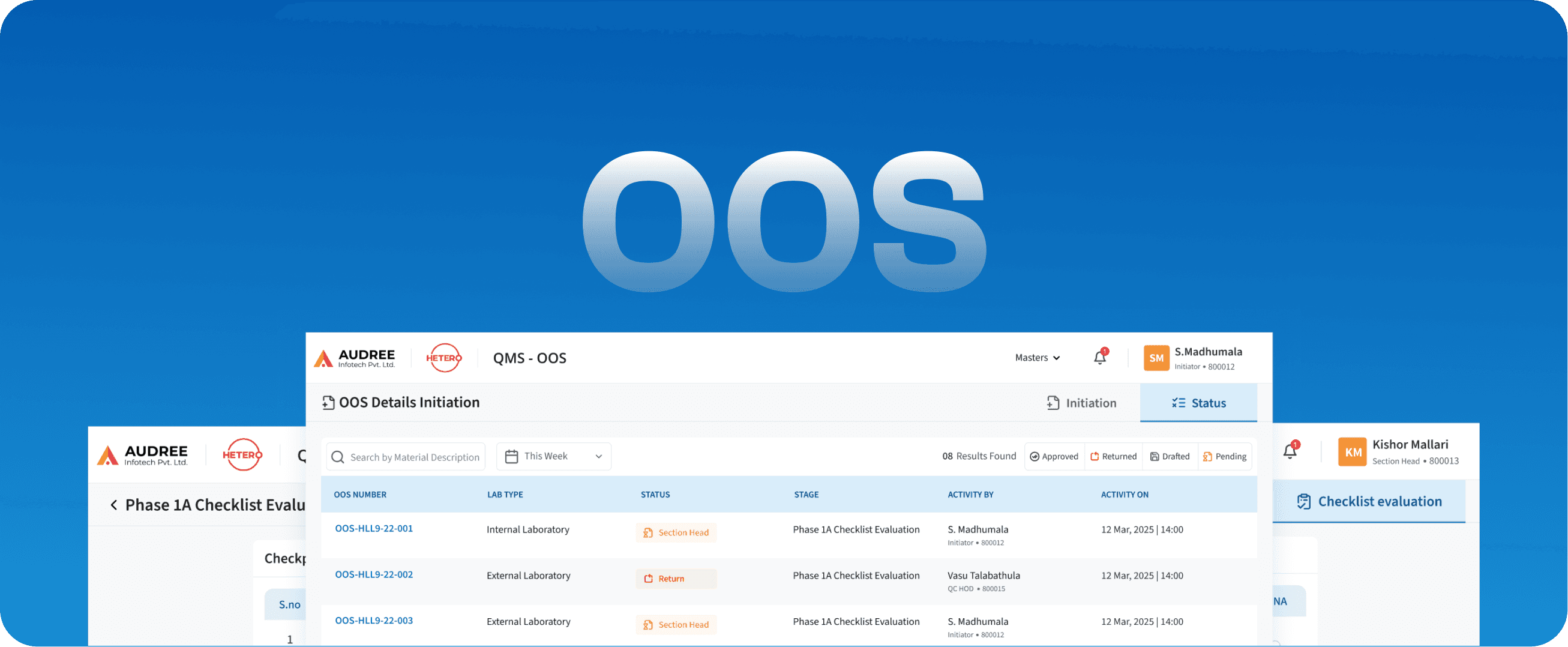
Out Of Specification
CMS
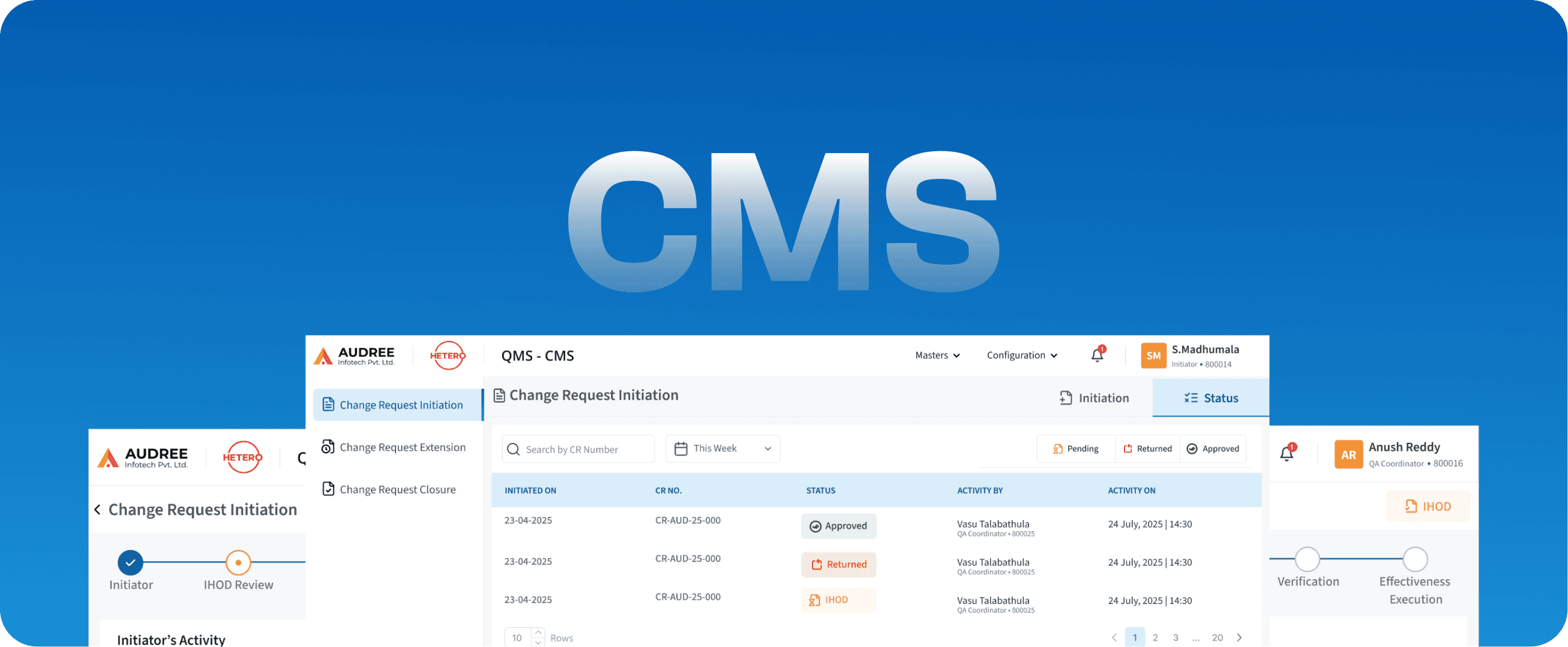
Change Management System
IMS
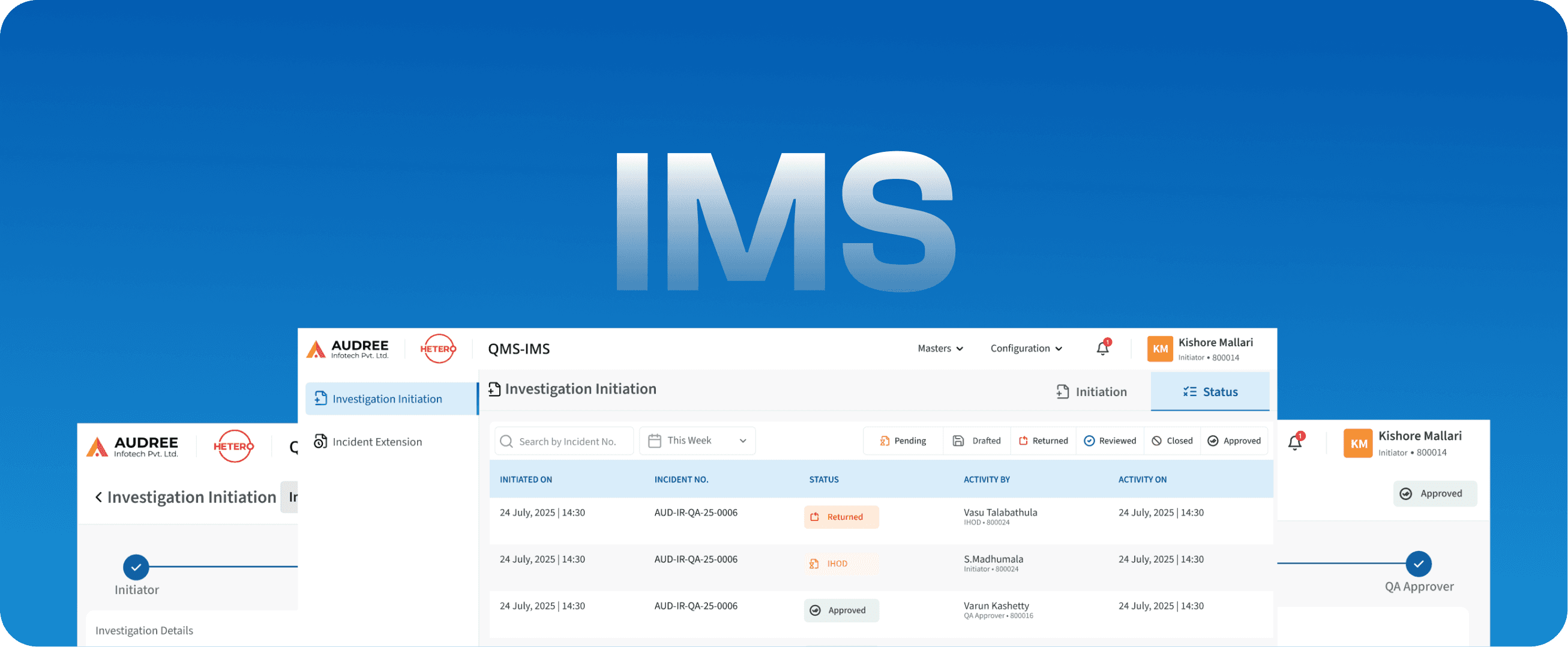
Incident Management System
BRMS-API
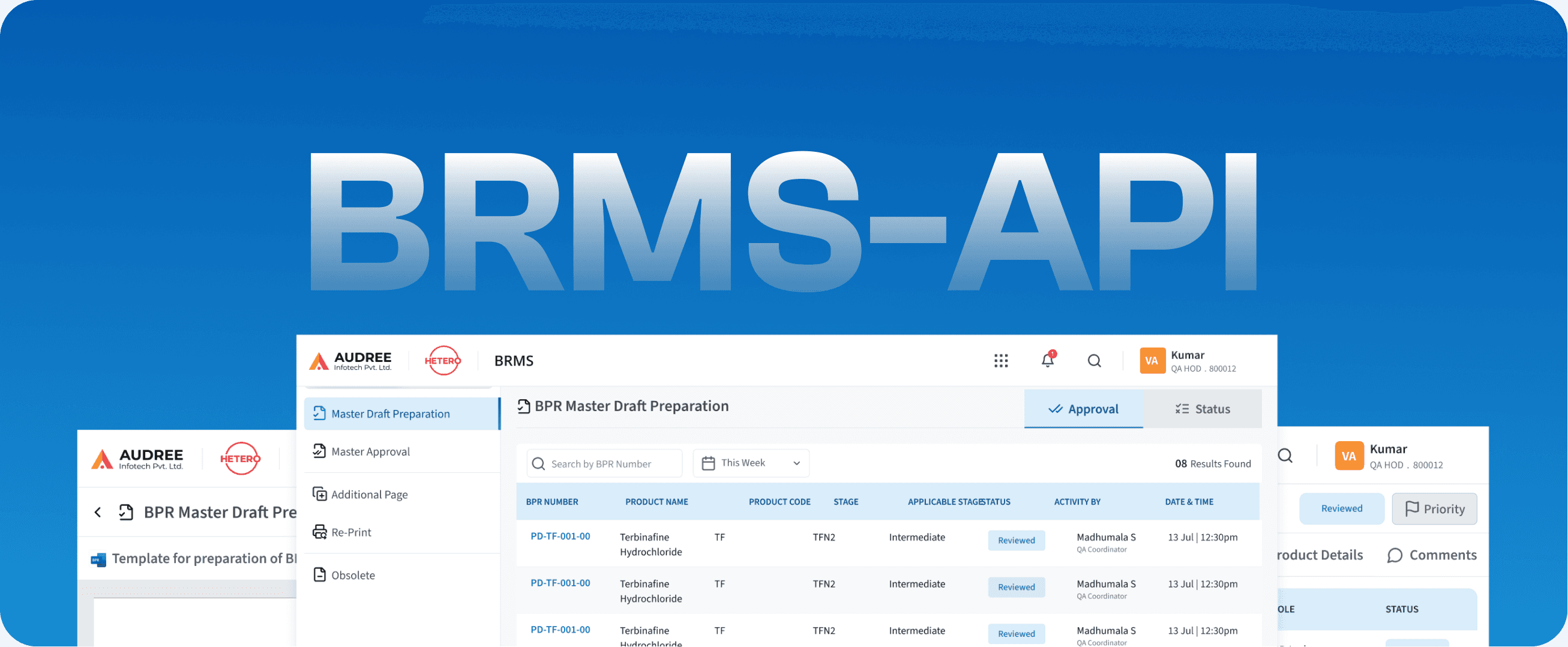
Batch Record- Active Pharmaceutical Ingredient
E-BRMS
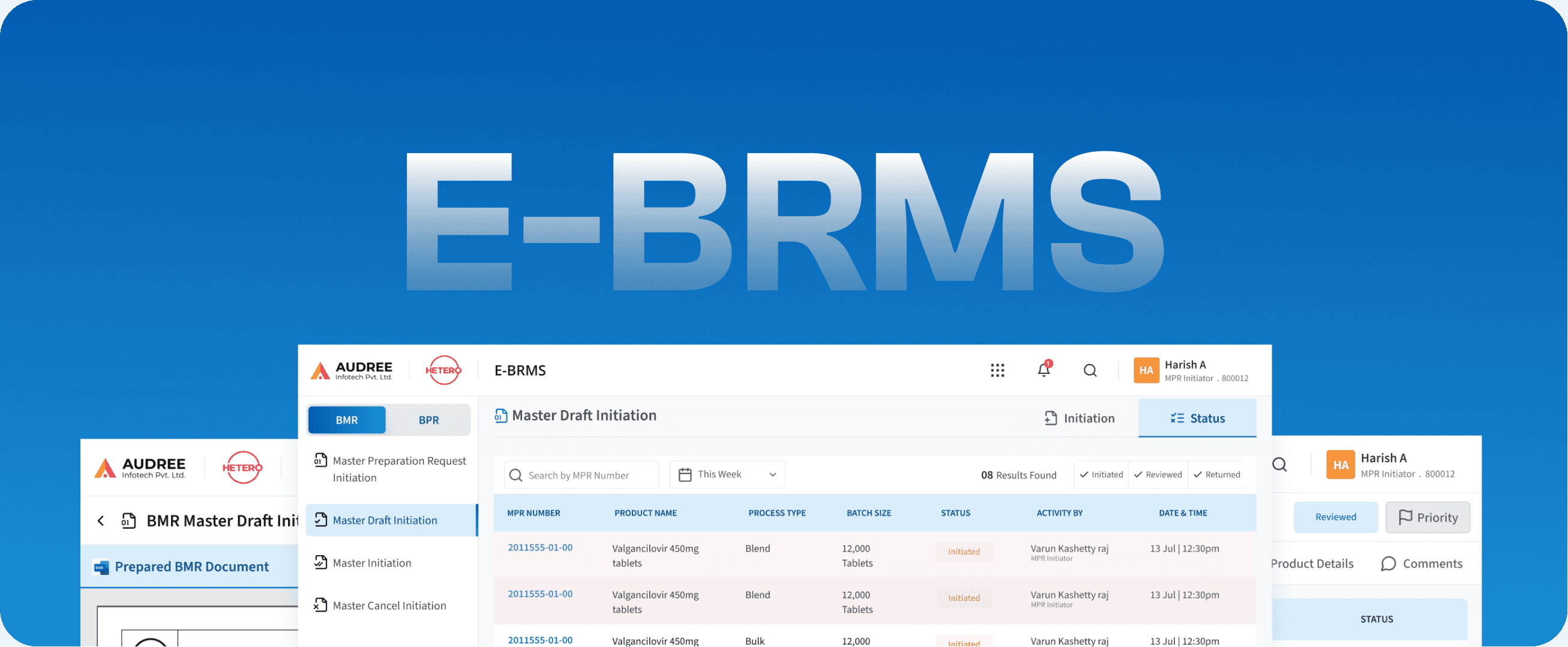
Batch Record Management System
RIMS
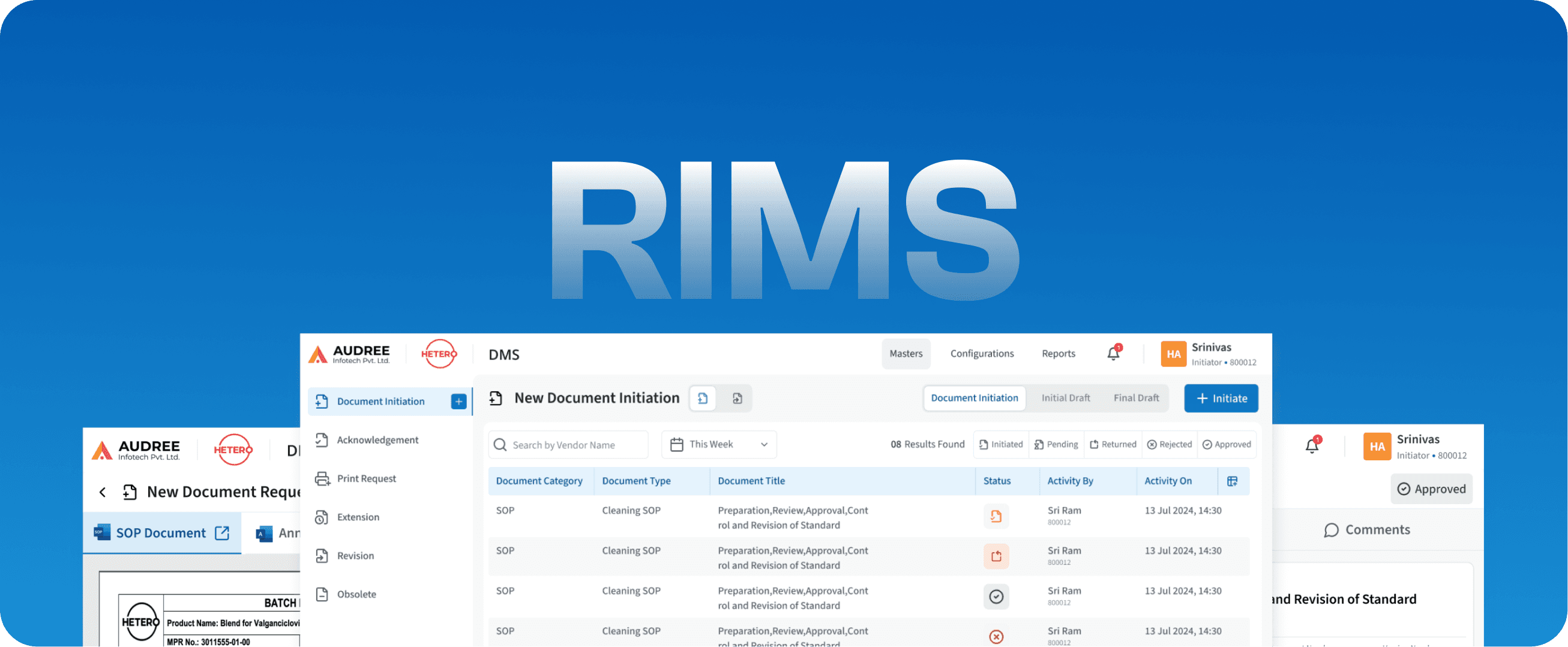
Regulatory Information Management System
APQR
Annual Product Quality Review
RCAI

Root Cause Analysis with Intelligence
LMS

Learning Management System
LIMS

Laboratory Information Management System
S & OP

Sales & Operations Planning
E-BMR

Batch Manufacturing Recall
RIMS
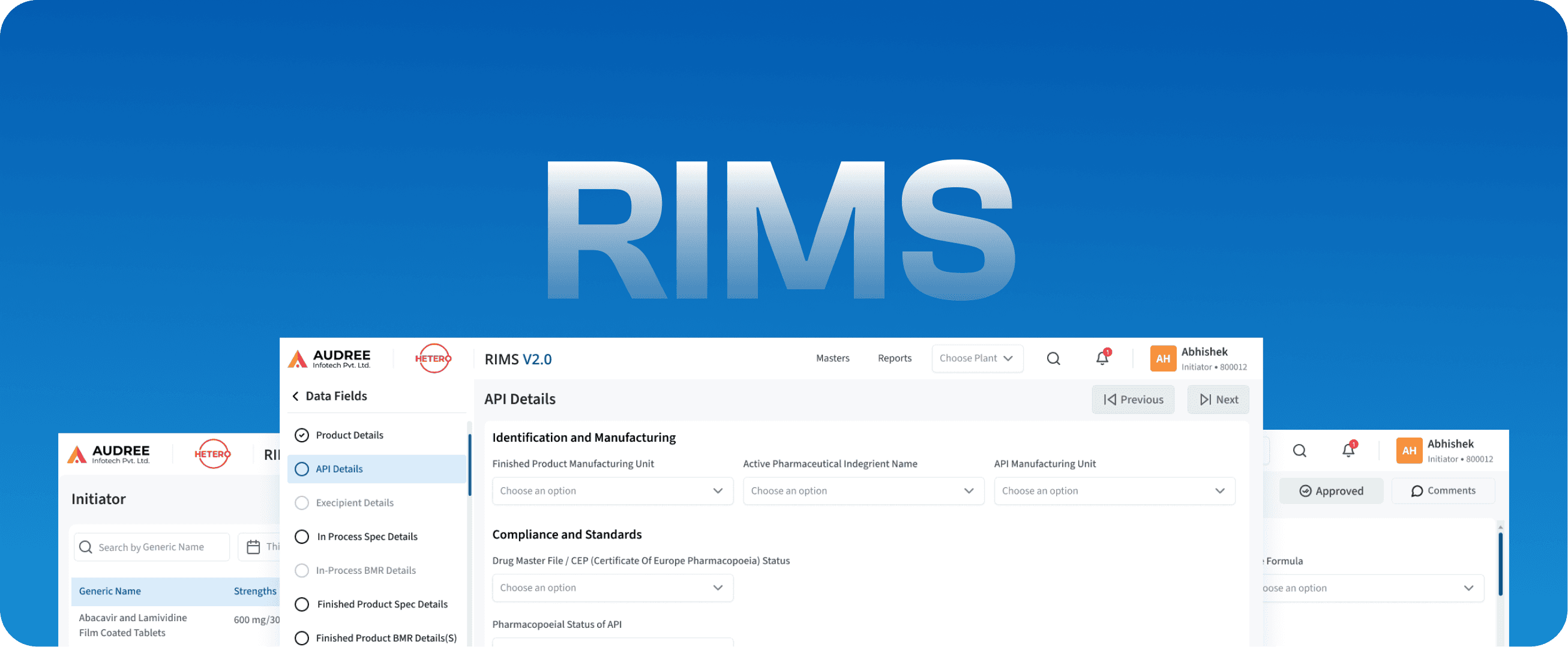
Regulatory Information Management Systems
IMS

Incident Management System
BRMS-API

Batch Record- Active Pharmaceutical Ingredient
E-BRMS

Batch Record Management System
APQR

Annual Product Quality Review
RIMS

Regulatory Information Management System
WMPS

Warehouse Management System
DMS

Document Management System
CMS

Change Management System
OOS

Out Of Specification
LIR-AER

Laboratory Information Record
Vendor Portal

Vendor Management System
QAS

Quality Agreement System
CAPA

Corrective And Preventive Actions

