Redesigning CMS Into a Guided, Compliant Workflow
Redesigning CMS Into a Guided, Compliant Workflow
Redesigning CMS Into a Guided, Compliant Workflow
About Project
About Project
About Project
The Change Management System (CMS) is a critical Quality module used in pharmaceutical environments to control, assess, and implement planned changes across manufacturing, equipment, documents, facilities, utilities, and quality processes.
CMS module lacked clarity, guidance, and structured workflows, leading to inconsistent documentation, delayed approvals, and manual coordination across teams.
Seeing these inefficiencies and inspired by the success of other redesigned QMS modules (OOS, IMS), Audree wanted CMS rebuilt into a clear, structured, digital workflow.
The Change Management System (CMS) is a critical Quality module used in pharmaceutical environments to control, assess, and implement planned changes across manufacturing, equipment, documents, facilities, utilities, and quality processes.
CMS module lacked clarity, guidance, and structured workflows, leading to inconsistent documentation, delayed approvals, and manual coordination across teams.
Seeing these inefficiencies and inspired by the success of other redesigned QMS modules (OOS, IMS), Audree wanted CMS rebuilt into a clear, structured, digital workflow.
About Project
Pharmaceutical
Team
Varun , S.Madhumala
Subscription Category
Quick win
Project start Year
August 2025
Core Business Challenges
Core Business Challenges
Core Business Challenges
Poor User Adoption Due to Outdated Experience
Poor User Adoption Due to Outdated Experience
Poor User Adoption Due to Outdated Experience
Cluttered, unclear screens made it hard to follow steps pushing teams to rely on emails and manual notes instead of the system.
Cluttered, unclear screens made it hard to follow steps pushing teams to rely on emails and manual notes instead of the system.
Cluttered, unclear screens made it hard to follow steps pushing teams to rely on emails and manual notes instead of the system.
Stuck in a Loop, Slowed by Inefficiency
Stuck in a Loop, Slowed by Inefficiency
Stuck in a Loop, Slowed by Inefficiency
Unclear steps and scattered screens made change requests hard to navigate, slowing assessments and delaying approvals.
Unclear steps and scattered screens made change requests hard to navigate, slowing assessments and delaying approvals.
Unclear steps and scattered screens made change requests hard to navigate, slowing assessments and delaying approvals.
Heavy Dependence on Manual Coordination
Heavy Dependence on Manual Coordination
Cross-functional teams relied on calls and emails , assessments weren’t clearly visible, causing repeated clarifications, slow approvals.
Cross-functional teams relied on calls and emails , assessments weren’t clearly visible, causing repeated clarifications, slow approvals.
Heavy Dependence on Manual Coordination
Cross-functional teams relied on calls and emails , assessments weren’t clearly visible, causing repeated clarifications, slow approvals.
Our Approach
Our Approach
Our Approach
Mapping User Flows to Uncover Hidden Gaps
We mapped the entire CMS workflow to uncover unclear steps, missing dependencies, and handover gaps. This helped us design a predictable, role-based flow that reduces confusion and rework.
Mapping User Flows to Uncover Hidden Gaps
We mapped the entire CMS workflow to uncover unclear steps, missing dependencies, and handover gaps. This helped us design a predictable, role-based flow that reduces confusion and rework.
Mapping User Flows to Uncover Hidden Gaps
We mapped the entire CMS workflow to uncover unclear steps, missing dependencies, and handover gaps. This helped us design a predictable, role-based flow that reduces confusion and rework.
Designing Change Management System Into Structured Workflow
Designing Change Management System Into Structured Workflow
Designing Change Management System Into Structured Workflow
We rebuilt the CMS lifecycle by turning a scattered, unclear change process into a guided, role-based workflow. Each stage from request initiation to closure now has clear action cues, structured documentation, and consistent inputs. With unified screens and improved stage visibility, teams no longer struggle with disconnected or confusing paths.
We rebuilt the CMS lifecycle by turning a scattered, unclear change process into a guided, role-based workflow. Each stage from request initiation to closure now has clear action cues, structured documentation, and consistent inputs. With unified screens and improved stage visibility, teams no longer struggle with disconnected or confusing paths.






Simplified CMS Initiation for Accurate Change Requests
Simplified CMS Initiation for Accurate Change Requests
Simplified CMS Initiation for Accurate Change Requests
We redesigned the CMS initiation flow to reflect how QA, Production, Engineering, and QC teams actually raise changes. Previously unclear fields and inconsistent inputs caused confusion, now every request starts with a clean, guided digital form.
Structured fields, smart validations, and grouped inputs ensure complete, accurate submissions reducing back-and-forth and speeding up the start of every change request.
We redesigned the CMS initiation flow to reflect how QA, Production, Engineering, and QC teams actually raise changes. Previously unclear fields and inconsistent inputs caused confusion, now every request starts with a clean, guided digital form.
Structured fields, smart validations, and grouped inputs ensure complete, accurate submissions reducing back-and-forth and speeding up the start of every change request.
We redesigned the CMS initiation flow to reflect how QA, Production, Engineering, and QC teams actually raise changes. Previously unclear fields and inconsistent inputs caused confusion, now every request starts with a clean, guided digital form.
Structured fields, smart validations, and grouped inputs ensure complete, accurate submissions reducing back-and-forth and speeding up the start of every change request.
Structured Review for Seamless Change Record
Structured Review for Seamless Change Record
Structured Review for Seamless Change Record
We redesigned the CMS interface with a clean stage-wise review experience, making every step easy to follow without searching or guessing.
Each role can clearly see:
What was completed in earlier steps
Impact assessment details, comments, and supporting evidence
Progress indicators with neat separation of actions
Current status and what comes next
This transforms a previously scattered review experience into structured change management.
We redesigned the CMS interface with a clean stage-wise review experience, making every step easy to follow without searching or guessing.
Each role can clearly see:
What was completed in earlier steps
Impact assessment details, comments, and supporting evidence
Progress indicators with neat separation of actions
Current status and what comes next
This transforms a previously scattered review experience into structured change management.
We redesigned the CMS interface with a clean stage-wise review experience, making every step easy to follow without searching or guessing.
Each role can clearly see:
What was completed in earlier steps
Impact assessment details, comments, and supporting evidence
Progress indicators with neat separation of actions
Current status and what comes next
This transforms a previously scattered review experience into structured change management.






Clear Visual Summaries for Confident Decision
Clear Visual Summaries for Confident Decision
Clear Visual Summaries for Confident Decision
We redesigned the Change Summary and Impact Overview to give teams a clear, visual snapshot of each change. Impact fields, linked documents, affected equipment, and risks are now displayed in a clean, structured layout.
With concise summaries and visual cues, approvers can review changes faster and make confident decisions without navigating multiple screens.
We redesigned the Change Summary and Impact Overview to give teams a clear, visual snapshot of each change. Impact fields, linked documents, affected equipment, and risks are now displayed in a clean, structured layout.
With concise summaries and visual cues, approvers can review changes faster and make confident decisions without navigating multiple screens.
We redesigned the Change Summary and Impact Overview to give teams a clear, visual snapshot of each change. Impact fields, linked documents, affected equipment, and risks are now displayed in a clean, structured layout.
With concise summaries and visual cues, approvers can review changes faster and make confident decisions without navigating multiple screens.


A Cleaner UI for Faster, Confident Decisions
A Cleaner UI for Faster, Confident Decisions
A Cleaner UI for Faster, Confident Decisions
The new CMS UI brings clarity to every step. With organised screens, structured data fields, and strong visual indicators, users can navigate confusion or unnecessary backtracking.
The new CMS UI brings clarity to every step. With organised screens, structured data fields, and strong visual indicators, users can navigate confusion or unnecessary backtracking.




Result That Redefined Change Management Lifecycle
Result That Redefined Change Management Lifecycle
Result That Redefined Change Management Lifecycle
Clearer workflows, structured assessments, and guided role based actions significantly improved change request quality, review speed, and cross-team coordination across all departments.
Clearer workflows, structured assessments, and guided role based actions significantly improved change request quality, review speed, and cross-team coordination across all departments.



Stronger Traceability & Control
Stronger Traceability & Control
Stronger Traceability & Control
Real-time visibility, structured forms, and consistent documentation ensured every assessment and approval was captured accurately reducing compliance gaps and making audits easier across facilities.
Real-time visibility, structured forms, and consistent documentation ensured every assessment and approval was captured accurately reducing compliance gaps and making audits easier across facilities.
Real-time visibility, structured forms, and consistent documentation ensured every assessment and approval was captured accurately reducing compliance gaps and making audits easier across facilities.

Faster Incident Closure
Guided digital steps replaced scattered screens and manual follow-ups, enabling faster assessments and approvals with linked evidence.

Reduced Follow-ups & Support Dependency
Unified screens and clear stage visibility reduced confusion and support dependency. All updates, now appear in one continuous flow eliminating backtracking and repeated clarifications.


Faster Incident Closure
Faster Incident Closure
Guided digital steps replaced scattered screens and manual follow-ups, enabling faster assessments and approvals with linked evidence.
Guided digital steps replaced scattered screens and manual follow-ups, enabling faster assessments and approvals with linked evidence.


Reduced Follow-ups & Support Dependency
Reduced Follow-up & Support Dependency
Unified screens and clear stage visibility reduced confusion and support dependency. All updates, now appear in one continuous flow eliminating backtracking and repeated clarifications.
Unified screens and clear stage visibility reduced confusion and support dependency. All updates, now appear in one continuous flow eliminating backtracking and repeated clarifications.
Deep-Dive Into More System
Deep-Dive Into More System
Deep-Dive Into More System
Browse every optimised Software and explore how legacy systems became intuitive.
Browse every optimised Software and explore how legacy systems became intuitive.
Browse every optimised Software and explore how legacy systems became intuitive.
RCAI

Root Cause Analysis with Intelligence
LMS
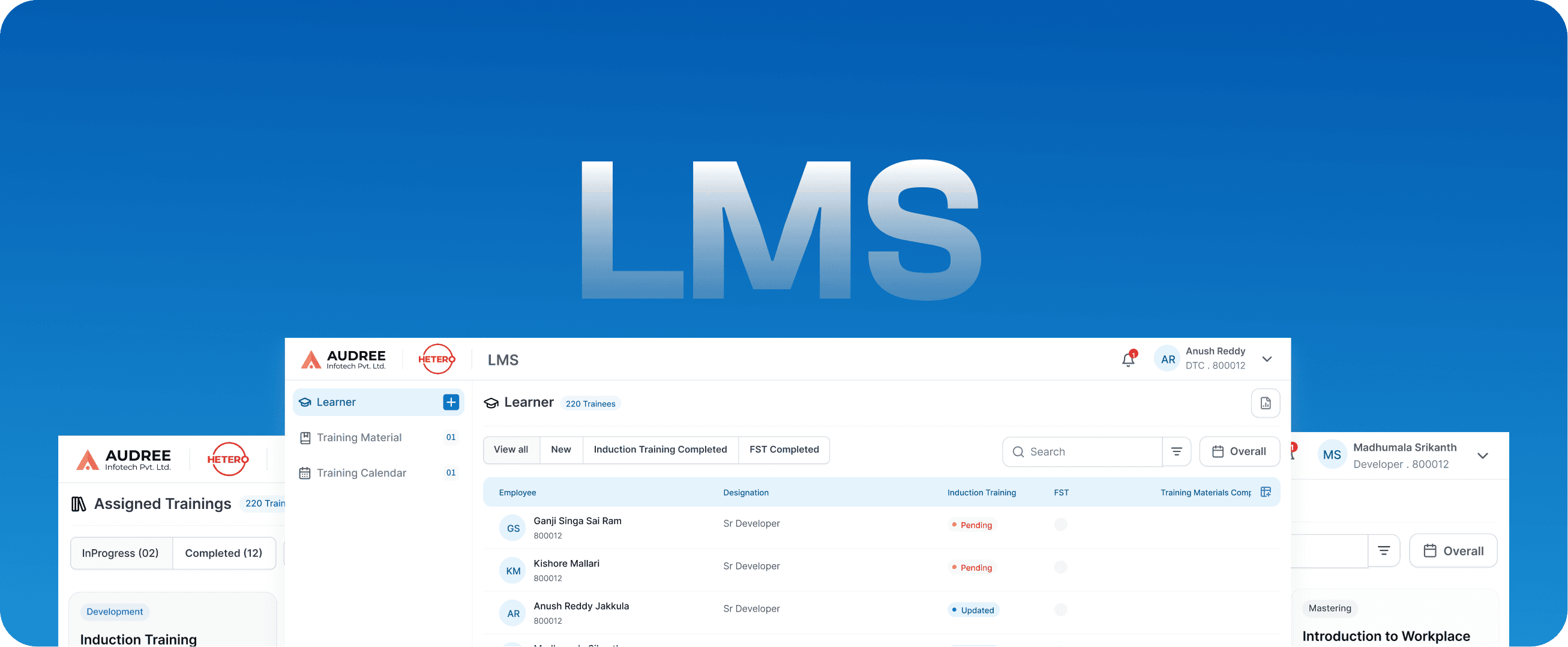
Learning Management System
LIMS
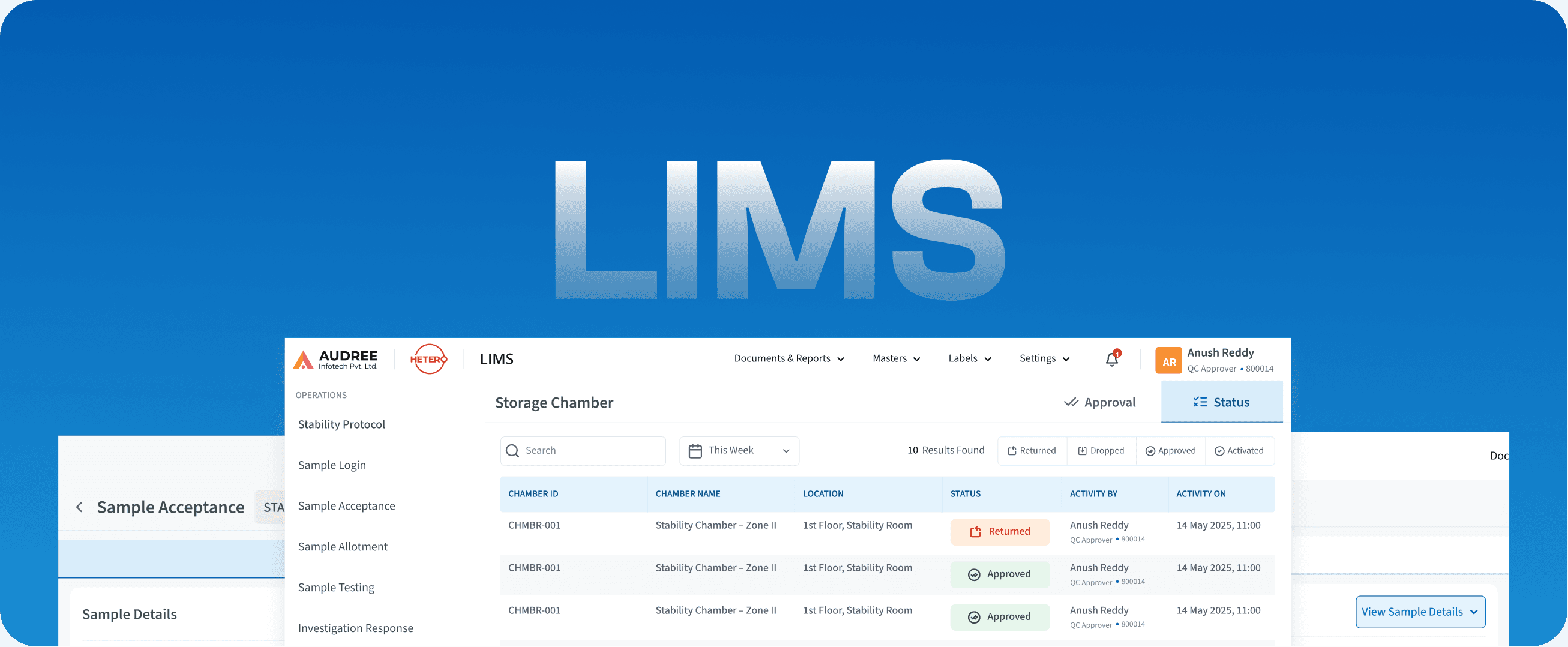
Laboratory Information Management System
S & OP
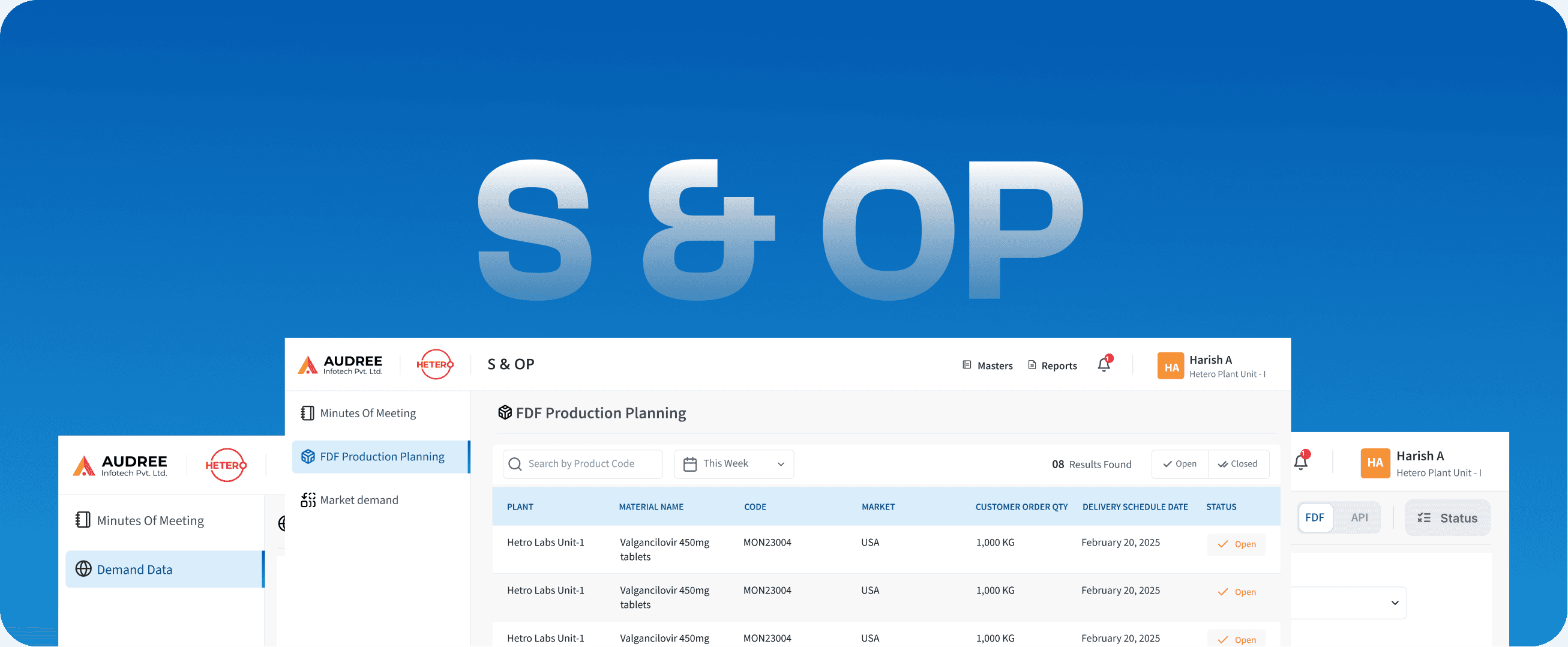
Sales & Operations Planning
E-BMR
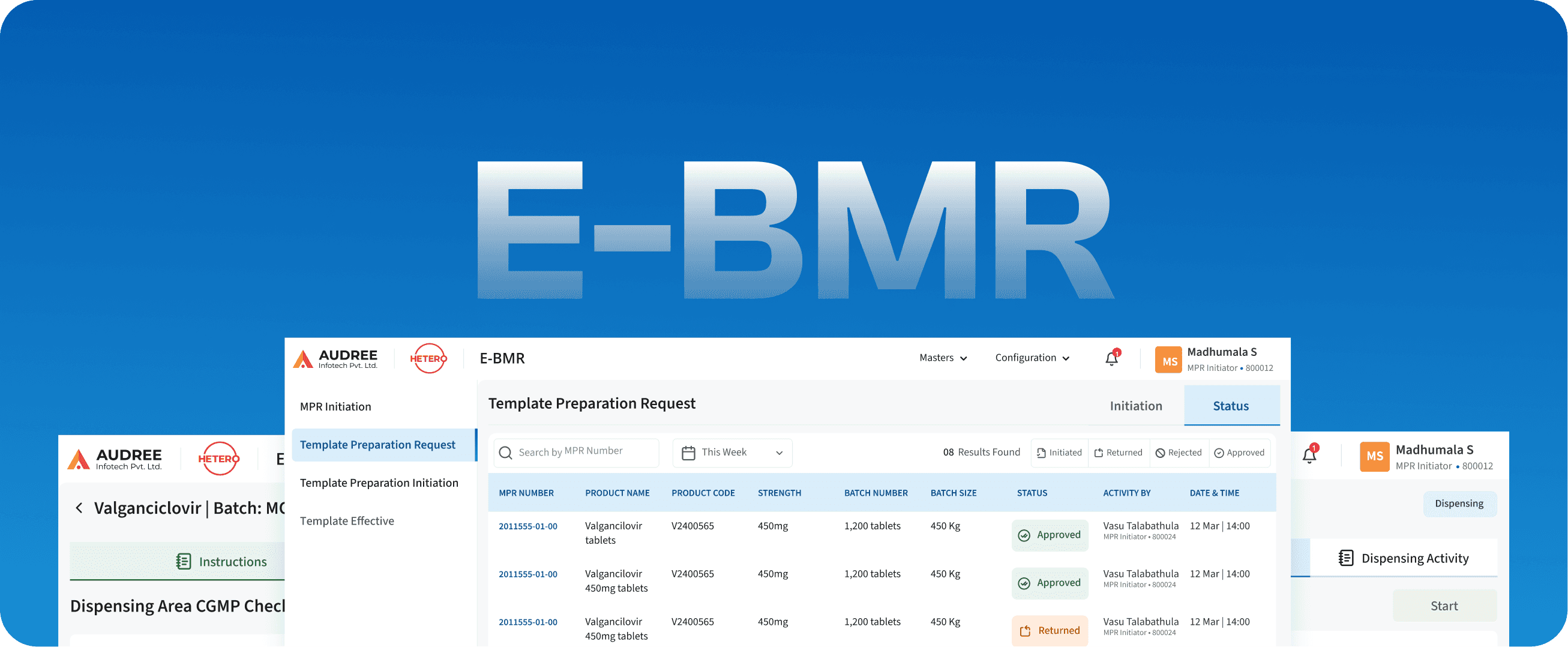
Batch Manufacturing Recall
WMPS

Warehouse Management System
DMS
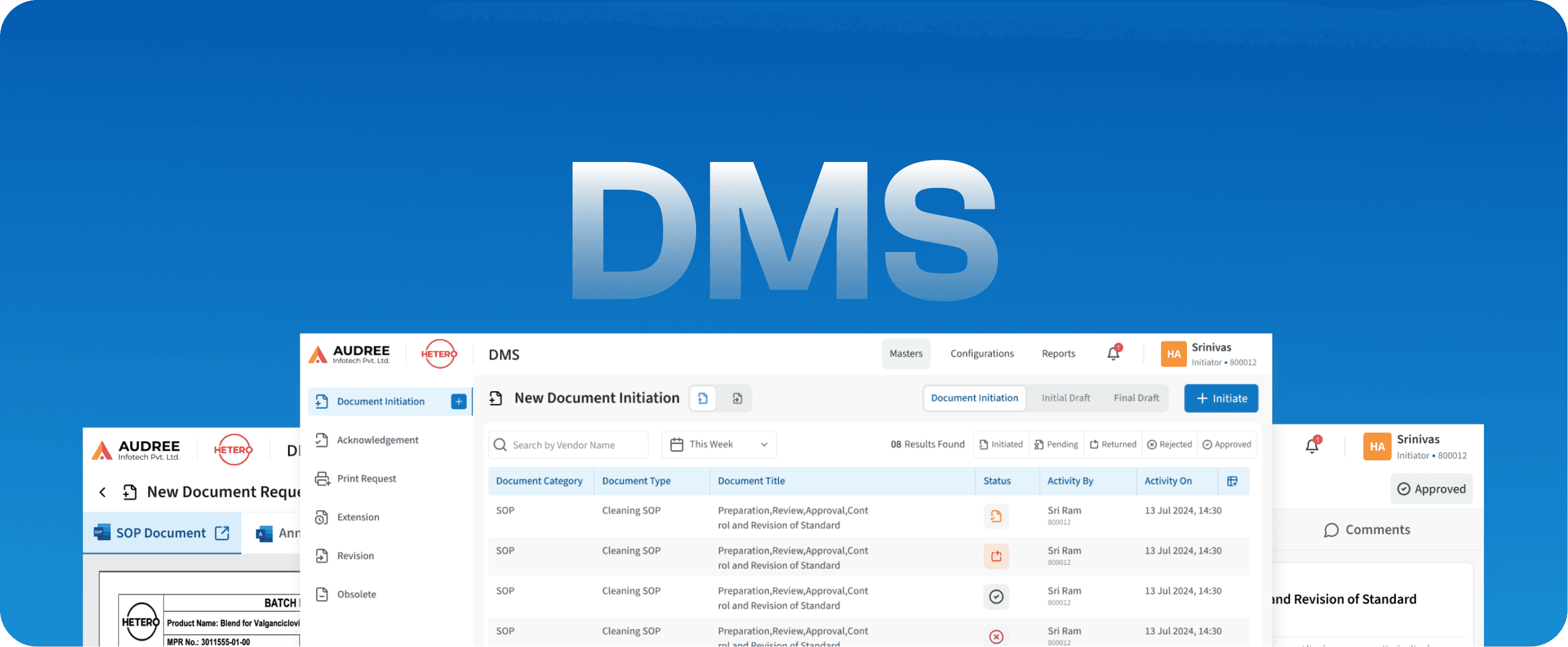
Document Management System
CAPA

Corrective And Preventive Actions
QAS

Quality Agreement System
Vendor Portal
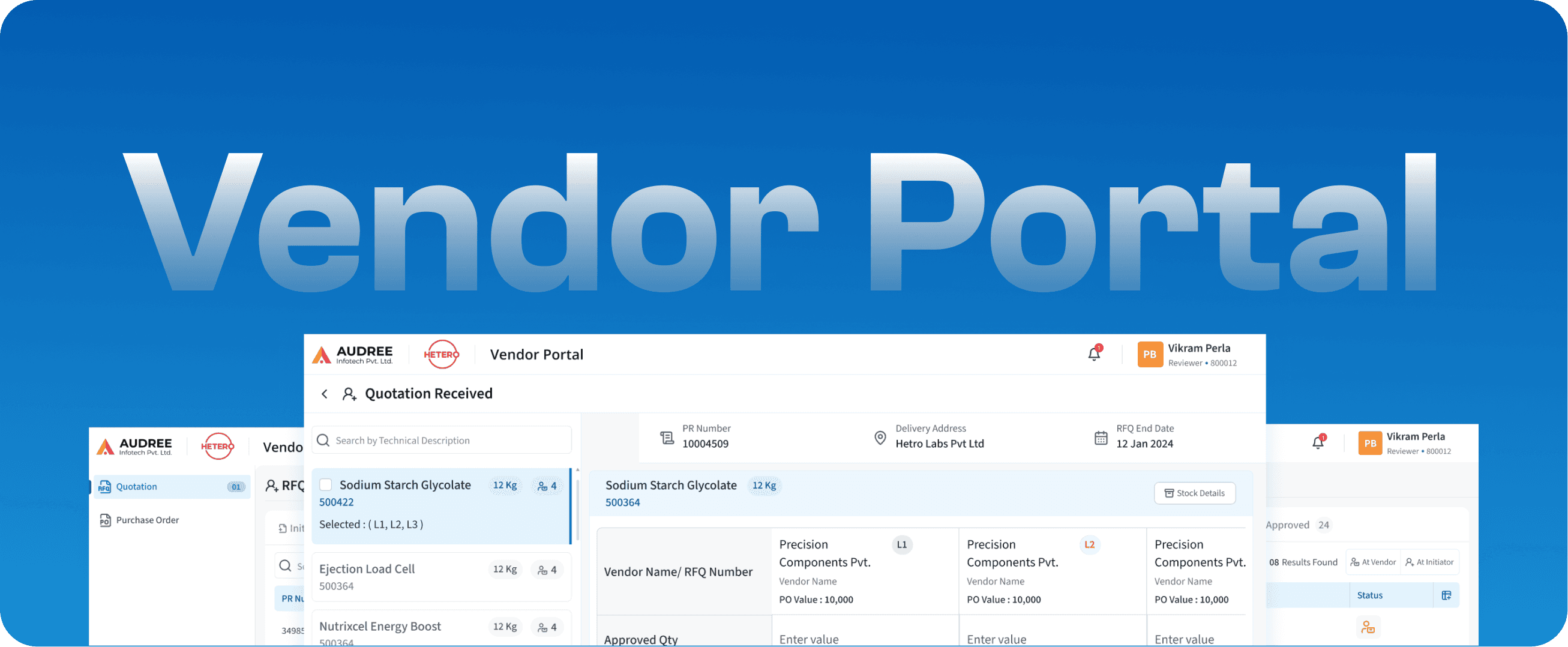
Vendor Management System
LIR-AER
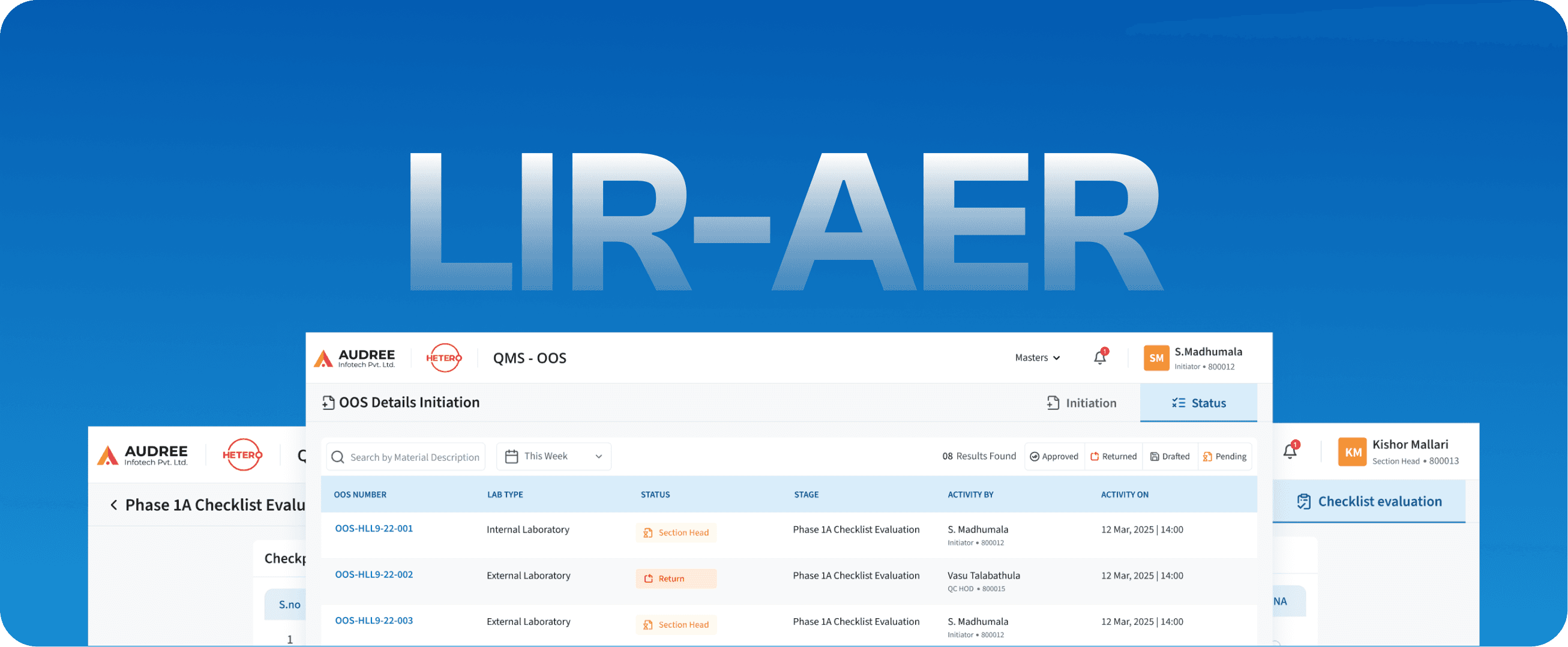
Laboratory Information Record
OOS
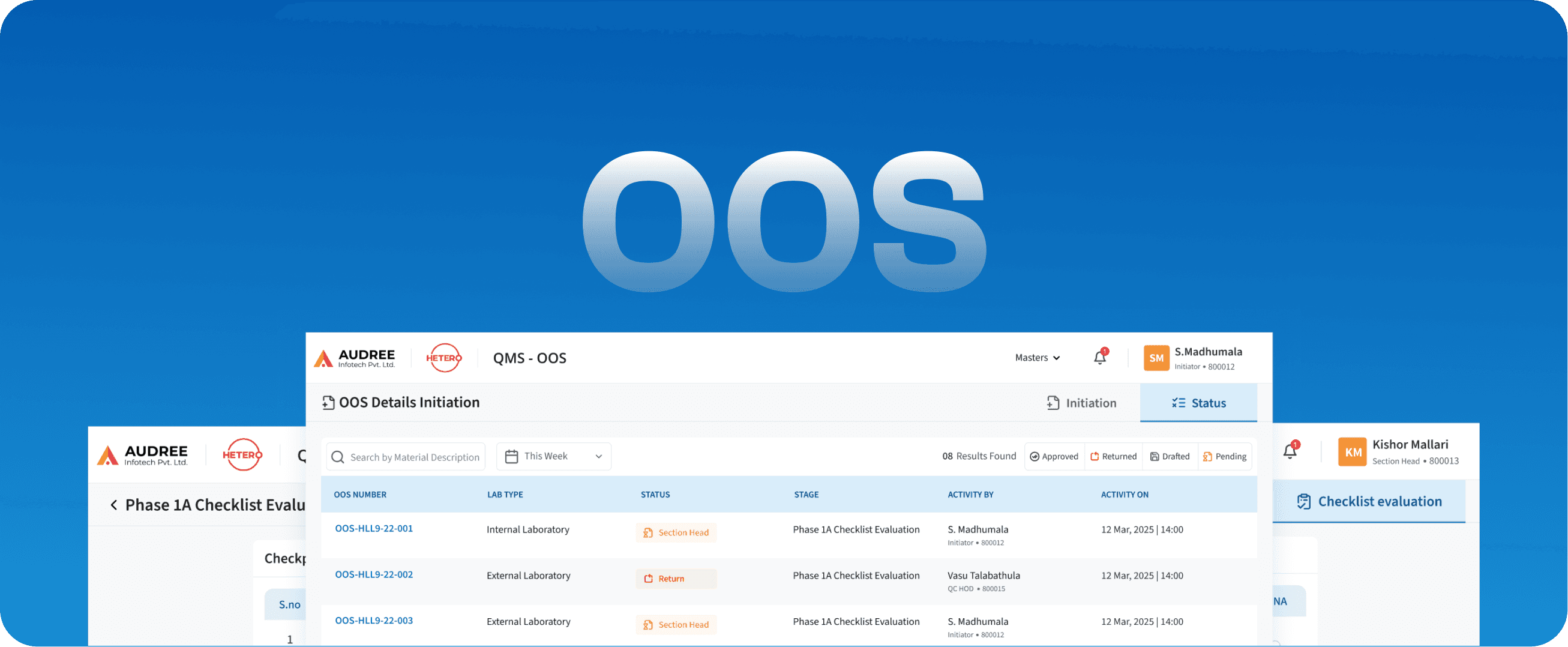
Out Of Specification
CMS
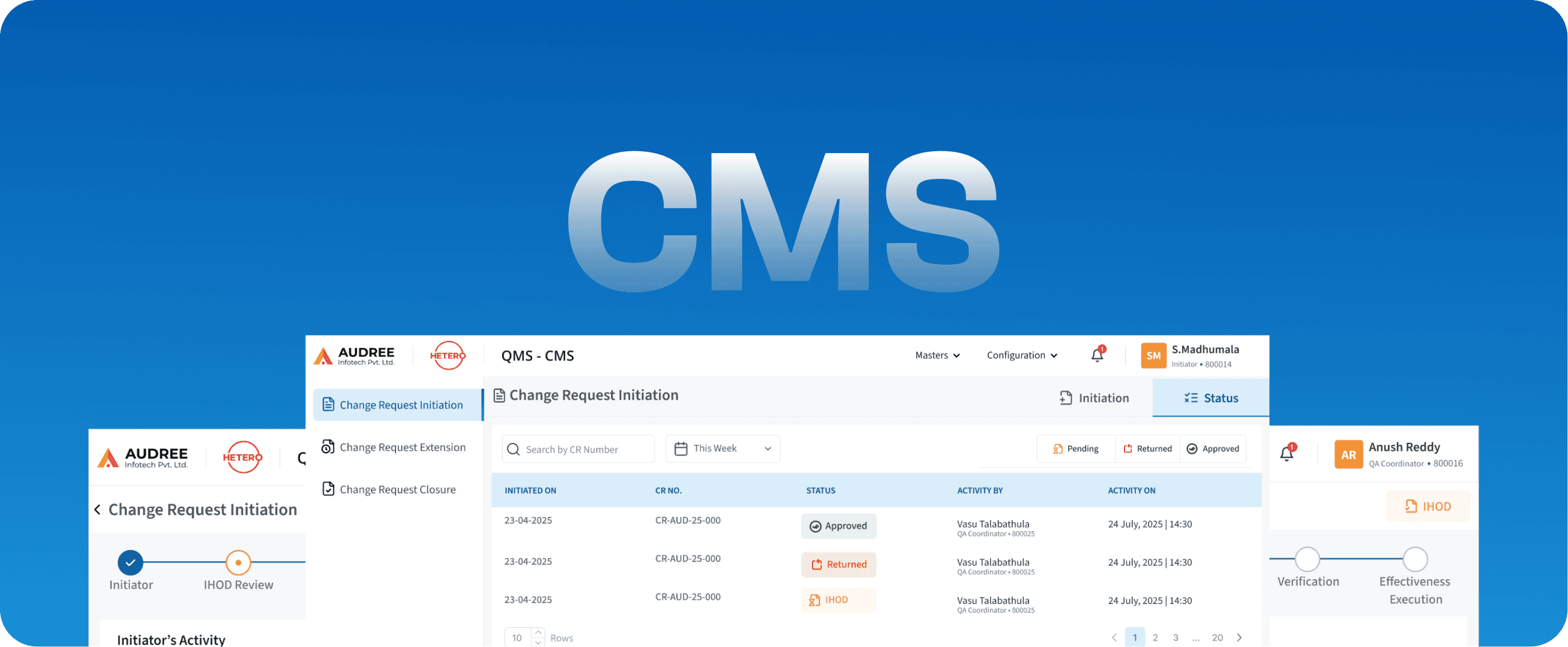
Change Management System
IMS
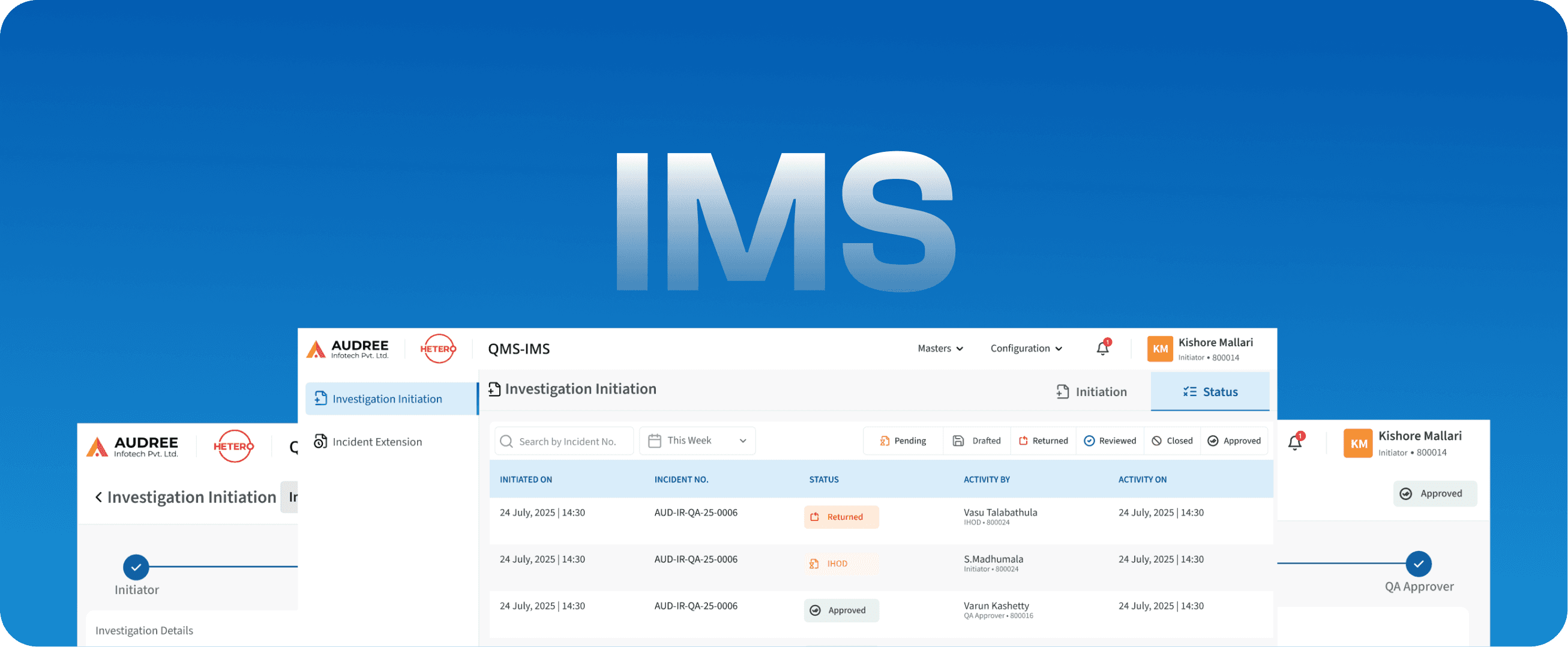
Incident Management System
BRMS-API
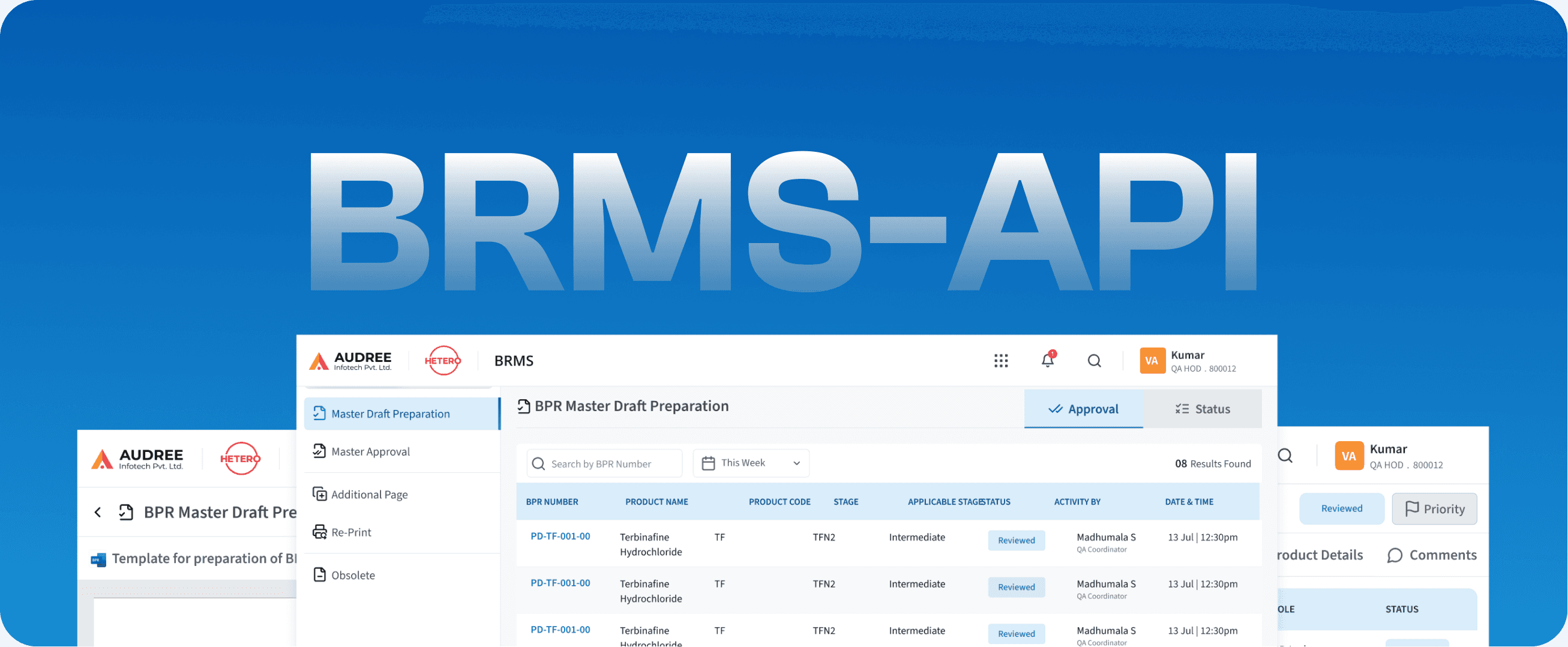
Batch Record- Active Pharmaceutical Ingredient
E-BRMS
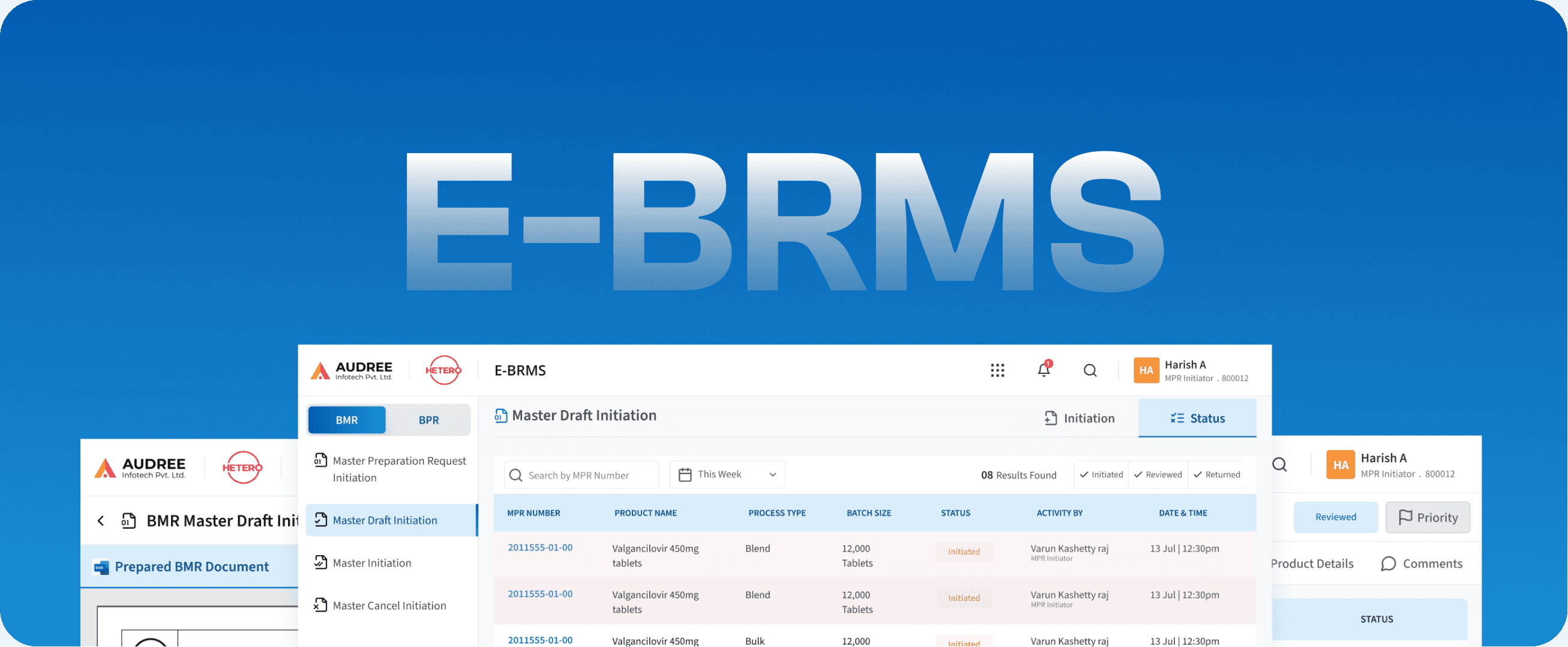
Batch Record Management System
RIMS
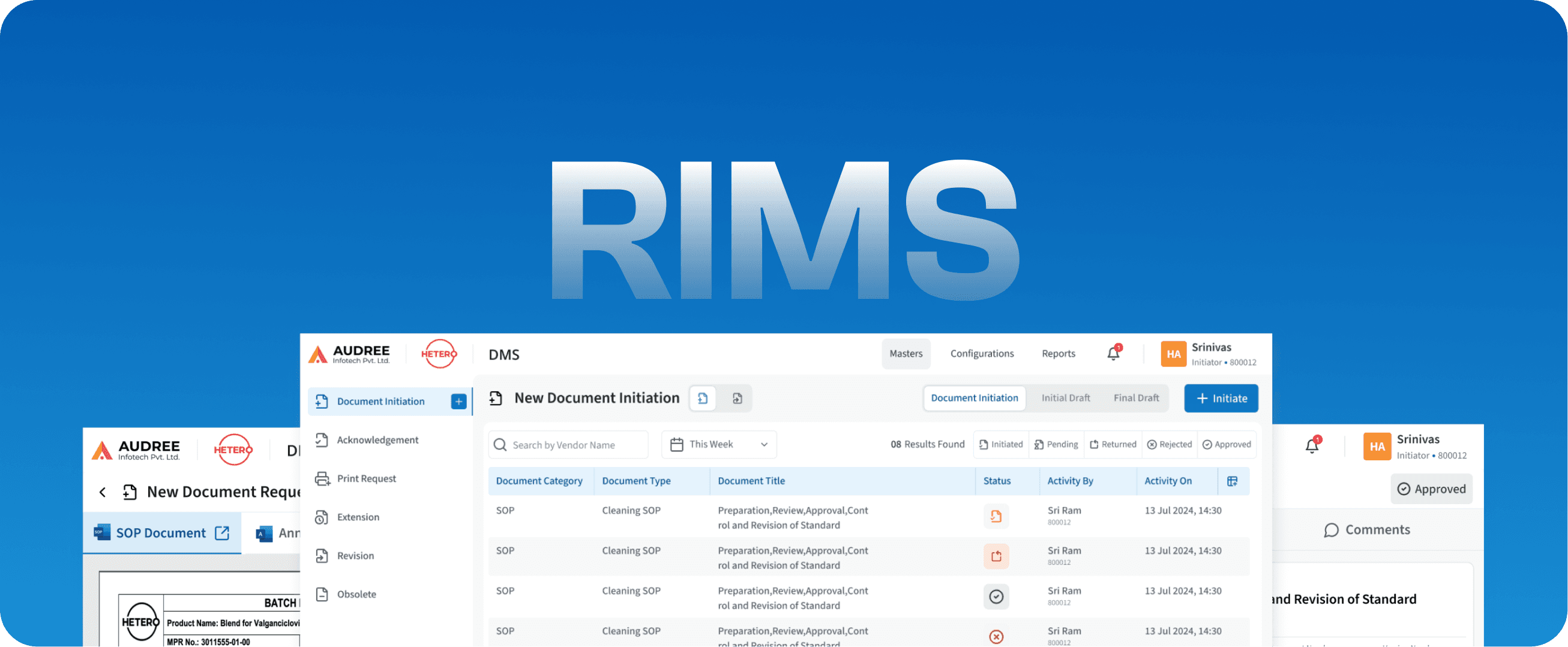
Regulatory Information Management System
APQR
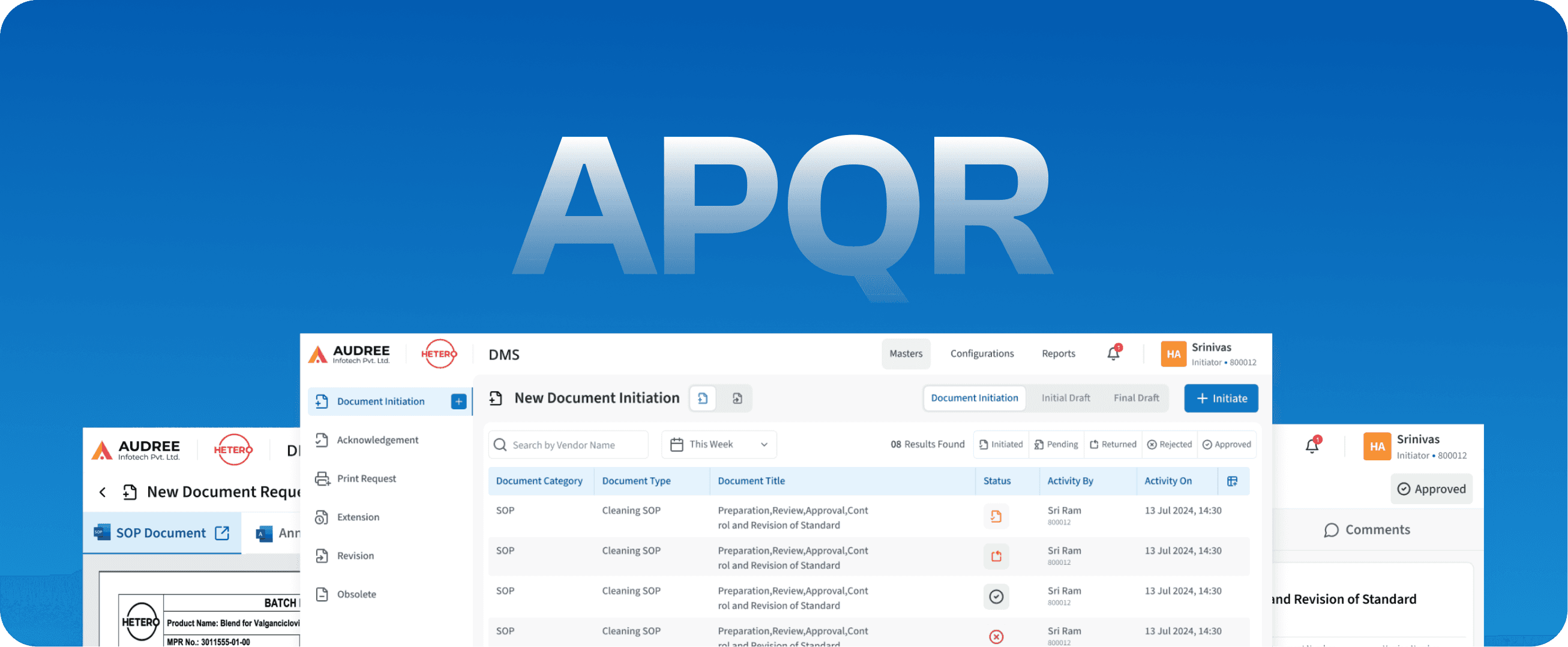
Annual Product Quality Review
RCAI

Root Cause Analysis with Intelligence
LMS

Learning Management System
LIMS

Laboratory Information Management System
S & OP

Sales & Operations Planning
E-BMR

Batch Manufacturing Recall
RIMS
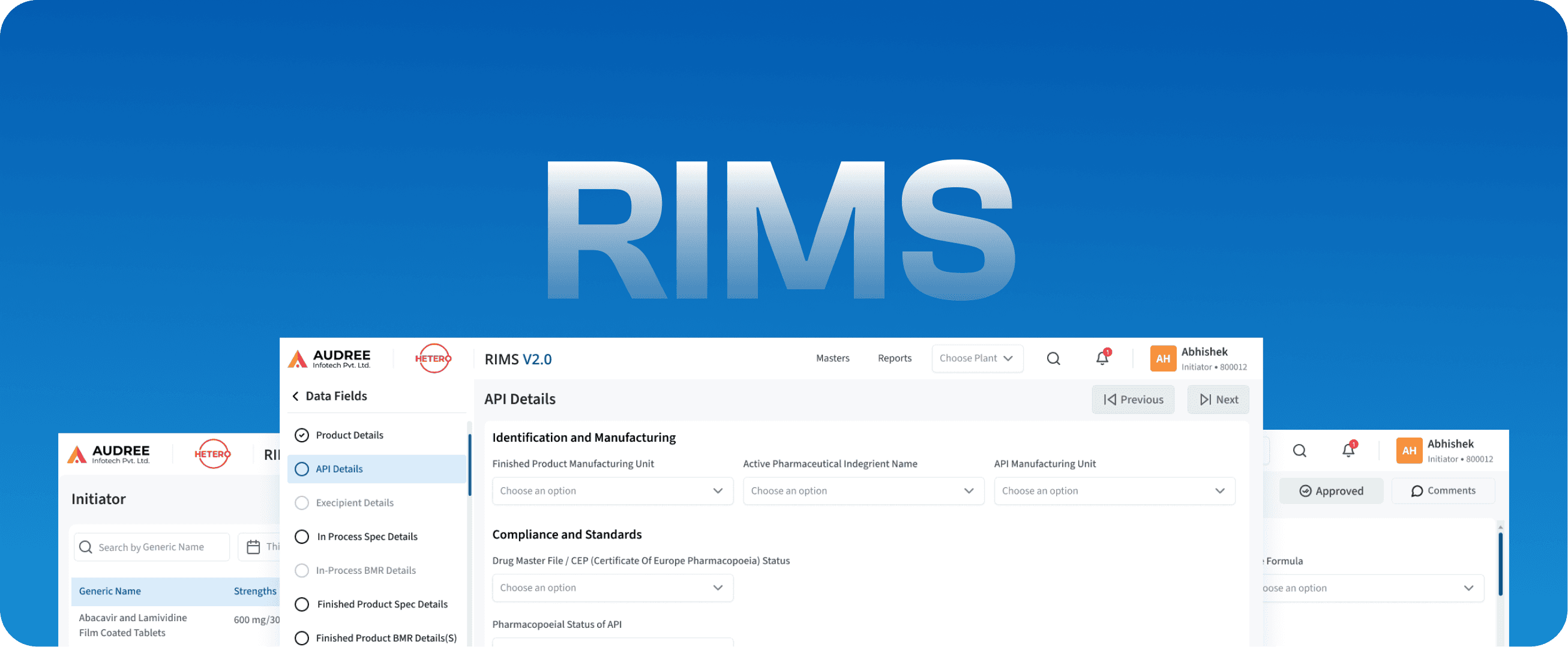
Regulatory Information Management Systems
IMS

Incident Management System
BRMS-API

Batch Record- Active Pharmaceutical Ingredient
E-BRMS

Batch Record Management System
APQR

Annual Product Quality Review
RIMS

Regulatory Information Management System
WMPS

Warehouse Management System
DMS

Document Management System
CMS

Change Management System
OOS

Out Of Specification
LIR-AER

Laboratory Information Record
Vendor Portal

Vendor Management System
QAS

Quality Agreement System
CAPA

Corrective And Preventive Actions

