Redesigning LMS Into a Simple, Compliant Training Workflow
Redesigning LMS Into a Simple, Compliant Training Workflow
Redesigning LMS Into a Simple, Compliant Training Workflow
About Project
About Project
About Project
The Learning Management System (LMS) is a critical compliance tool in pharma, used to manage training assignments, certifications, assessments, and competency tracking across roles.
However, the existing LMS was difficult to navigate, heavily manual, and hard to track leading to missed trainings, repeated follow-ups, and audit risk.
Audree wanted to redesign LMS into a clear, role-based, and audit-ready digital workflow that simplifies training management for employees, reviewers, and administrators while ensuring regulatory compliance.
The Learning Management System (LMS) is a critical compliance tool in pharma, used to manage training assignments, certifications, assessments, and competency tracking across roles.
However, the existing LMS was difficult to navigate, heavily manual, and hard to track leading to missed trainings, repeated follow-ups, and audit risk.
Audree wanted to redesign LMS into a clear, role-based, and audit-ready digital workflow that simplifies training management for employees, reviewers, and administrators while ensuring regulatory compliance.
About Project
Pharmaceutical
Team
Anush Reddy, S.Madhumala
Subscription Category
Quick win
Project start Year
August 2025
Core Business Challenges
Core Business Challenges
Core Business Challenges
Poor User Adoption Due to Outdated Experience
Poor User Adoption Due to Outdated Experience
Poor User Adoption Due to Outdated Experience
Complex lists and unclear status indicators made it hard for users to understand assigned trainings and next steps.
Complex lists and unclear status indicators made it hard for users to understand assigned trainings and next steps.
Complex lists and unclear status indicators made it hard for users to understand assigned trainings and next steps.
Slowed by Process Inefficiency
Slowed by Process Inefficiency
Slowed by Process Inefficiency
Training data lived across spreadsheets and emails, making it hard to track completions, reviews, and closures on time.
Training data lived across spreadsheets and emails, making it hard to track completions, reviews, and closures on time.
Training data lived across spreadsheets and emails, making it hard to track completions, reviews, and closures on time.
Heavy Dependence on Manual Coordination
Heavy Dependence on Manual Coordination
Limited visibility into training progress forced repeated reminders, emails, and calls between learners, reviewers, and QA.
Limited visibility into training progress forced repeated reminders, emails, and calls between learners, reviewers, and QA.
Heavy Dependence on Manual Coordination
Limited visibility into training progress forced repeated reminders, emails, and calls between learners, reviewers, and QA.
Our Approach
Our Approach
Our Approach
Mapping User Flows to Uncover Hidden Gaps
We mapped the complete LMS lifecycle from training creation and assignment to completion, review, approval, and audit closure.
This helped uncover fragmented tracking, repeated manual follow-ups, unclear reviewer ownership, and limited visibility across employees, managers, and QA teams.
Mapping User Flows to Uncover Hidden Gaps
We mapped the complete LMS lifecycle from training creation and assignment to completion, review, approval, and audit closure.
This helped uncover fragmented tracking, repeated manual follow-ups, unclear reviewer ownership, and limited visibility across employees, managers, and QA teams.
Mapping User Flows to Uncover Hidden Gaps
We mapped the complete LMS lifecycle from training creation and assignment to completion, review, approval, and audit closure.
This helped uncover fragmented tracking, repeated manual follow-ups, unclear reviewer ownership, and limited visibility across employees, managers, and QA teams.
Designing LMS Into a Guided Training Workflow
Designing LMS Into a Guided Training Workflow
Designing LMS Into a Guided Training Workflow
We redesigned the pharma LMS from fragmented training records and manual tracking into a structured, role-based learning workflow. Clear training assignments, status visibility, and approval flows improved compliance tracking, reduced follow-ups, and enabled teams to complete, review, and certify trainings with confidence across the organization.
We redesigned the pharma LMS from fragmented training records and manual tracking into a structured, role-based learning workflow. Clear training assignments, status visibility, and approval flows improved compliance tracking, reduced follow-ups, and enabled teams to complete, review, and certify trainings with confidence across the organization.



Training Reviews Made Simple
Training Reviews Made Simple
Training Reviews Made Simple
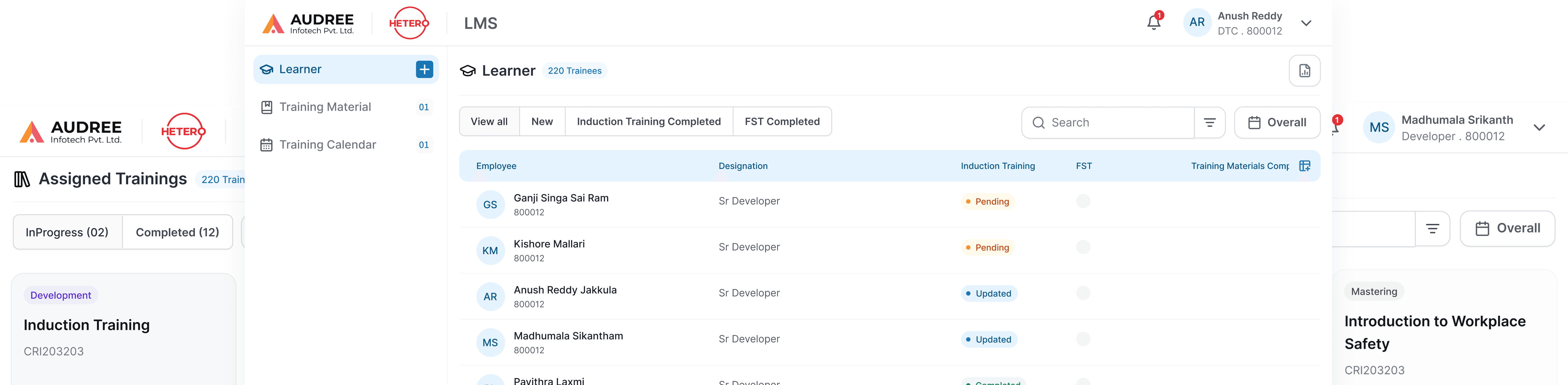
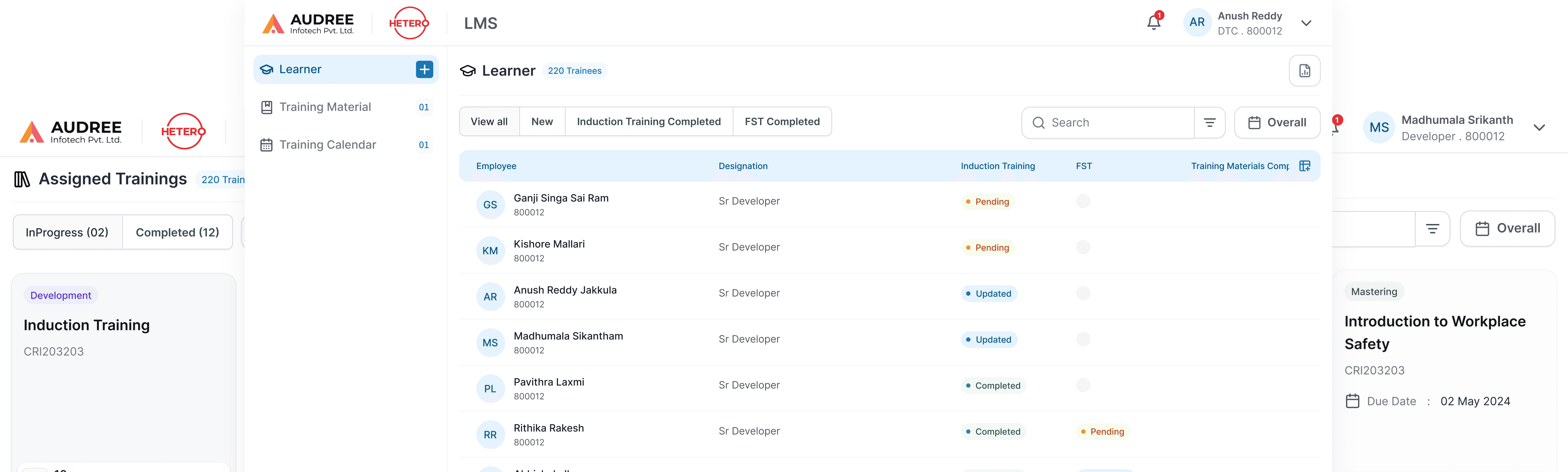
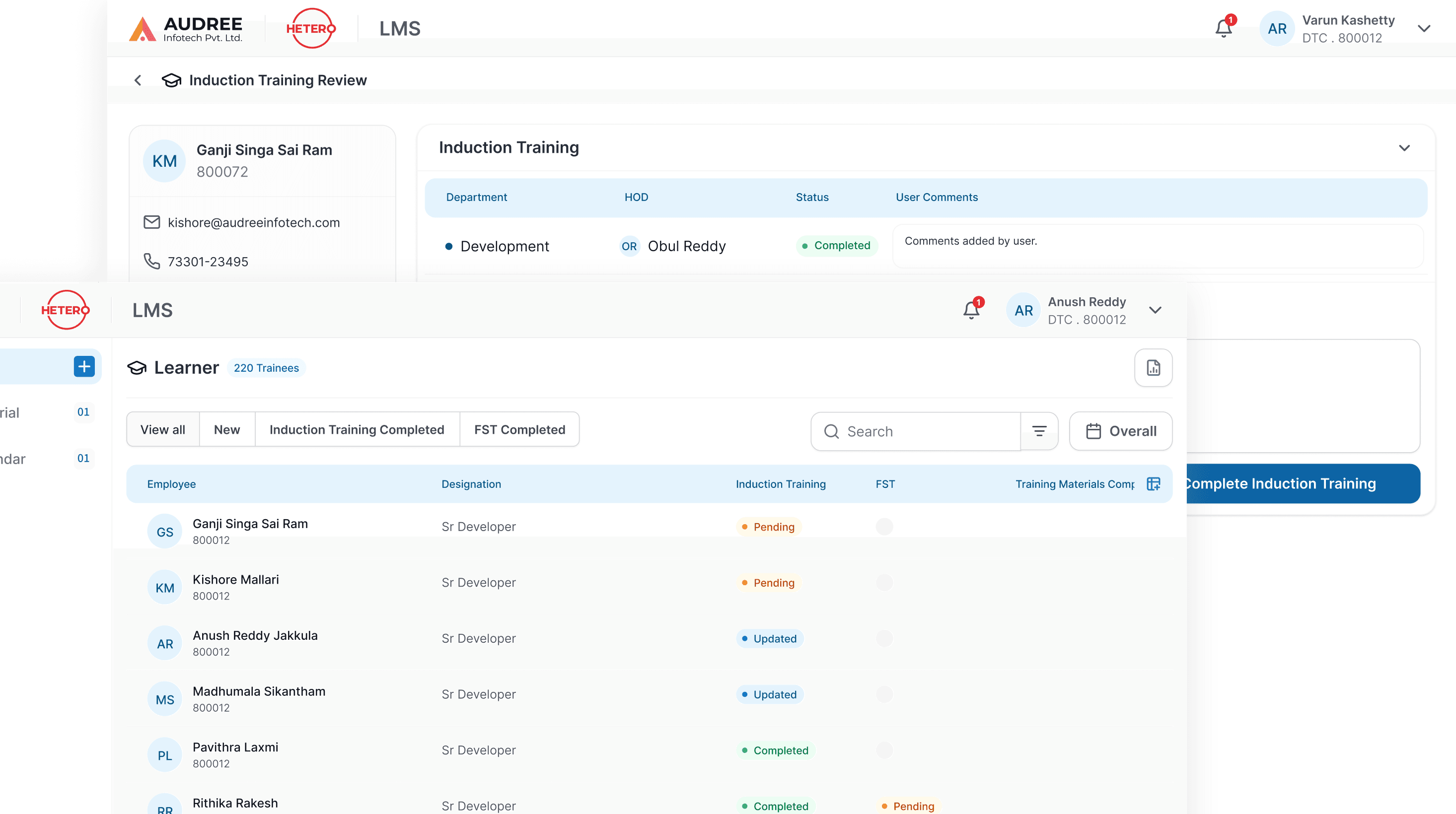
We redesigned the LMS training review flow to give managers and QA teams clear visibility into induction and role-based trainings. Admins can assign training materials directly to users, everything managed in one unified screen eliminating emails and spreadsheets.
Clear trainee lists, status indicators, and reviewer actions make it easy to track pending trainings, validate completions, and close reviews efficiently, ensuring timely compliance with minimal manual effort.
We redesigned the LMS training review flow to give managers and QA teams clear visibility into induction and role-based trainings. Admins can assign training materials directly to users, everything managed in one unified screen eliminating emails and spreadsheets.
Clear trainee lists, status indicators, and reviewer actions make it easy to track pending trainings, validate completions, and close reviews efficiently, ensuring timely compliance with minimal manual effort.
We redesigned the LMS training review flow to give managers and QA teams clear visibility into induction and role-based trainings. Admins can assign training materials directly to users, everything managed in one unified screen eliminating emails and spreadsheets.
Clear trainee lists, status indicators, and reviewer actions make it easy to track pending trainings, validate completions, and close reviews efficiently, ensuring timely compliance with minimal manual effort.
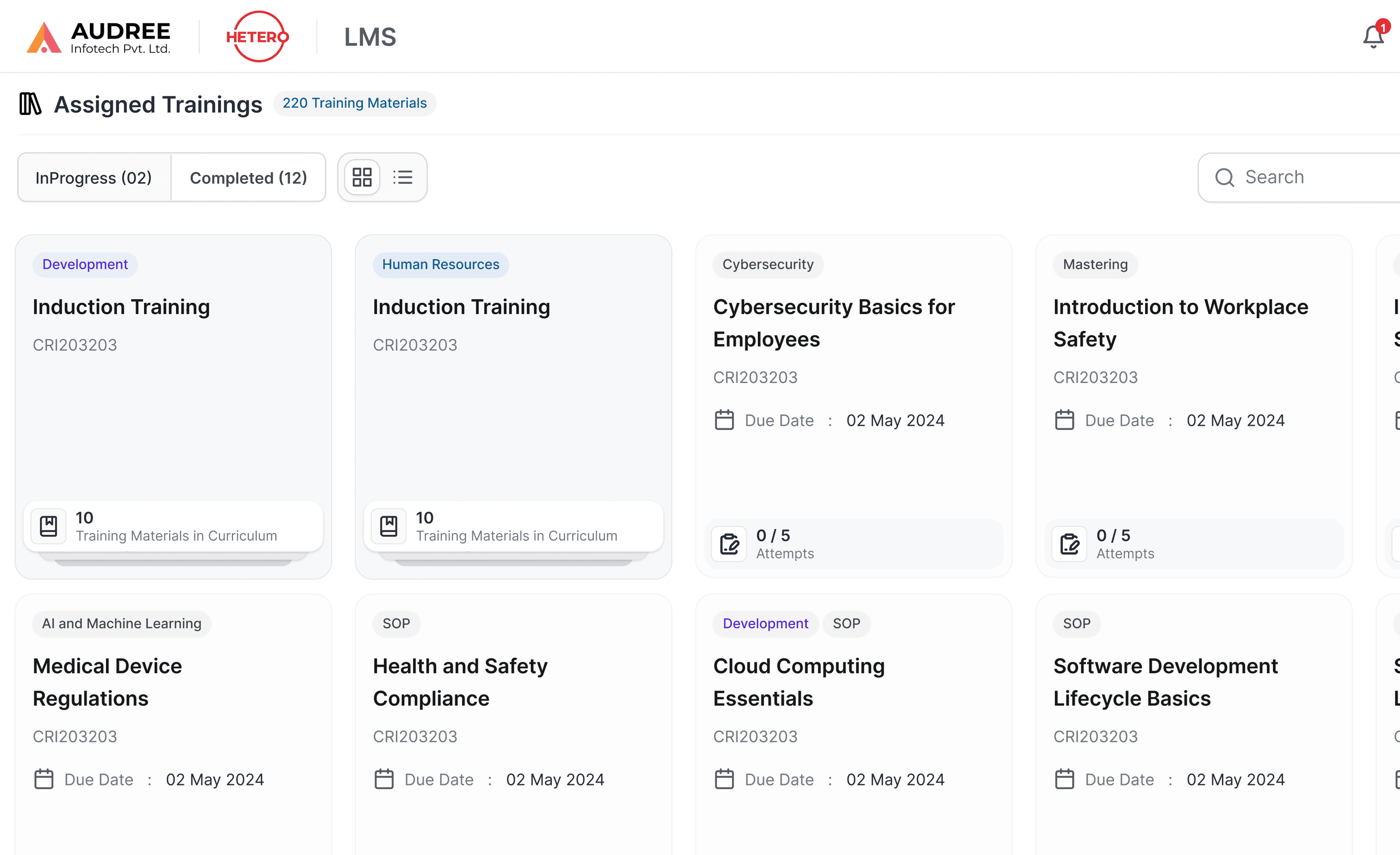
Structured View for Assigned Trainings
Structured View for Assigned Trainings
Structured View for Assigned Trainings
Users can view all assigned training programs in one organized space, with clear grouping by department, category, and completion status. Card-based view make it easy to understand what’s in progress, what’s completed, and what needs attention next.
Clear labels, due dates, material counts, and progress indicators help users plan learning without confusion. This reduces dependency on reminders or manual tracking and ensures employees can complete required trainings on time with full clarity.
Users can view all assigned training programs in one organized space, with clear grouping by department, category, and completion status. Card-based view make it easy to understand what’s in progress, what’s completed, and what needs attention next.
Clear labels, due dates, material counts, and progress indicators help users plan learning without confusion. This reduces dependency on reminders or manual tracking and ensures employees can complete required trainings on time with full clarity.
Users can view all assigned training programs in one organized space, with clear grouping by department, category, and completion status. Card-based view make it easy to understand what’s in progress, what’s completed, and what needs attention next.
Clear labels, due dates, material counts, and progress indicators help users plan learning without confusion. This reduces dependency on reminders or manual tracking and ensures employees can complete required trainings on time with full clarity.


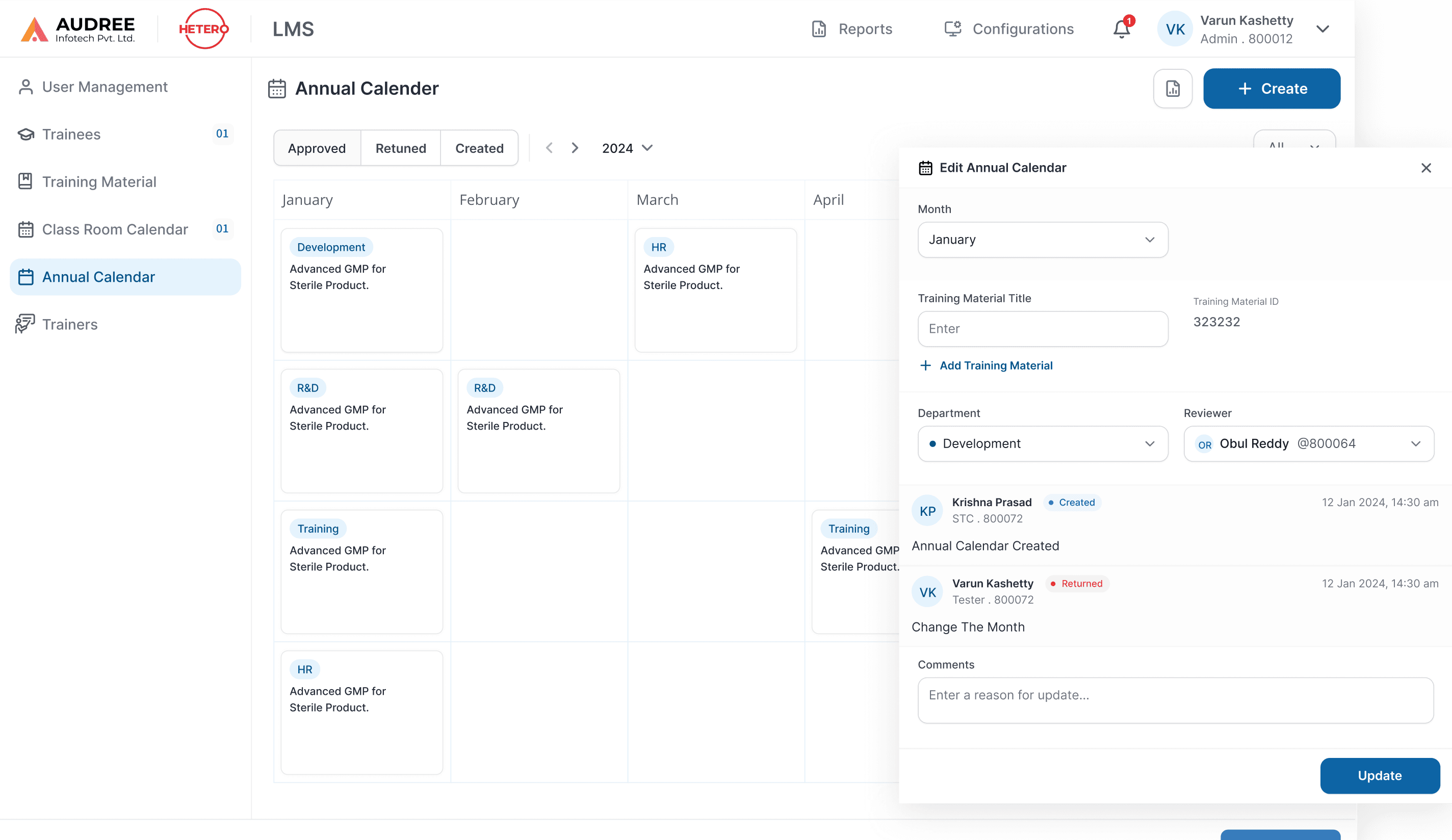


Clear Training Calendar for Planned Compliance
Clear Training Calendar for Planned Compliance
Clear Training Calendar for Planned Compliance
The Annual Training Calendar gives a month-wise view of all department trainings, showing scheduled, approved, returned, and pending items. Teams can plan inductions, GMP, SOP, and role-based trainings without spreadsheets or offline tracking.
Clear calendar cards and status indicators show training coverage at a glance, while inline edits and approvals keep plans accurate, auditable, and on track.
The Annual Training Calendar gives a month-wise view of all department trainings, showing scheduled, approved, returned, and pending items. Teams can plan inductions, GMP, SOP, and role-based trainings without spreadsheets or offline tracking.
Clear calendar cards and status indicators show training coverage at a glance, while inline edits and approvals keep plans accurate, auditable, and on track.
The Annual Training Calendar gives a month-wise view of all department trainings, showing scheduled, approved, returned, and pending items. Teams can plan inductions, GMP, SOP, and role-based trainings without spreadsheets or offline tracking.
Clear calendar cards and status indicators show training coverage at a glance, while inline edits and approvals keep plans accurate, auditable, and on track.
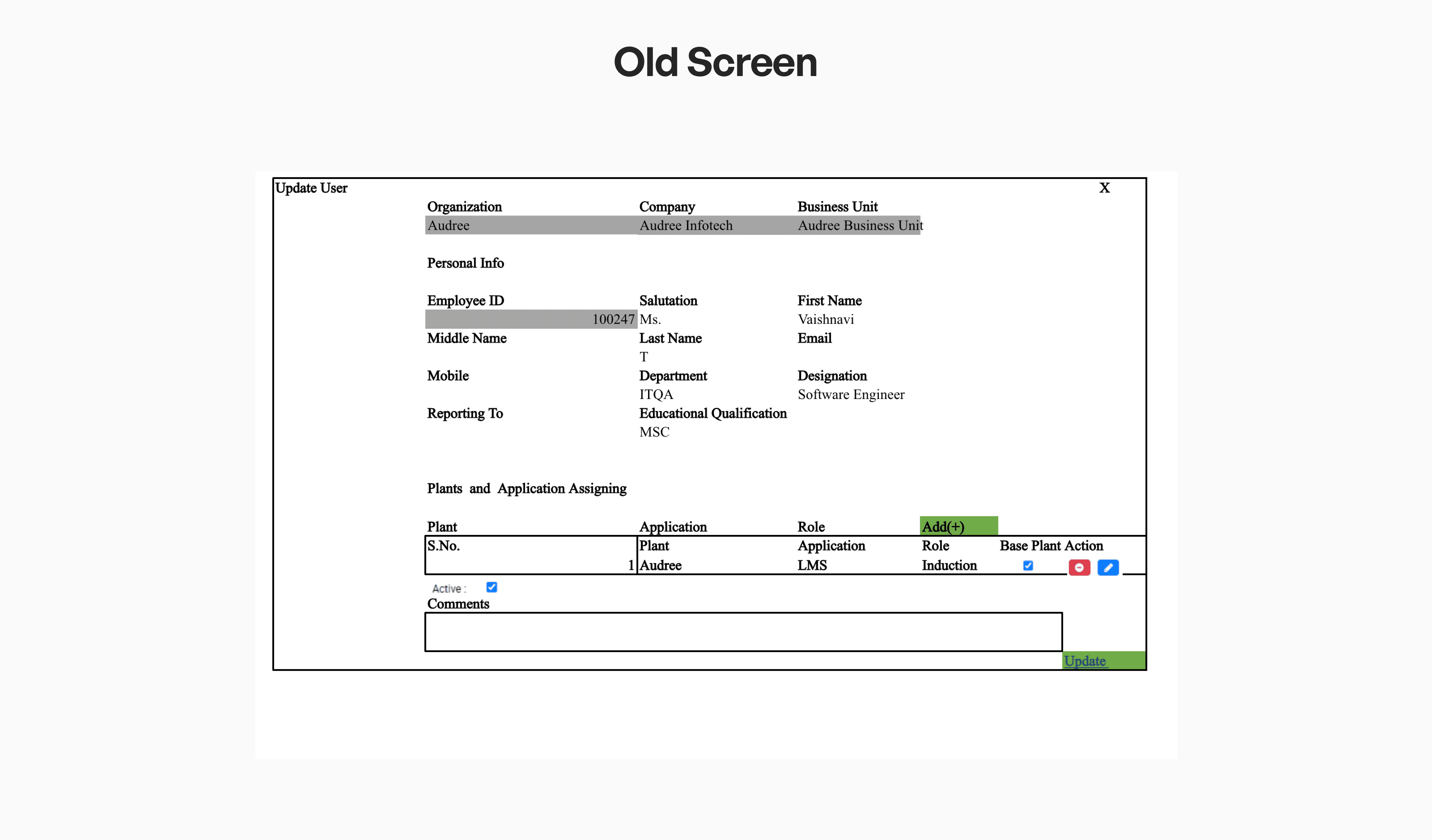
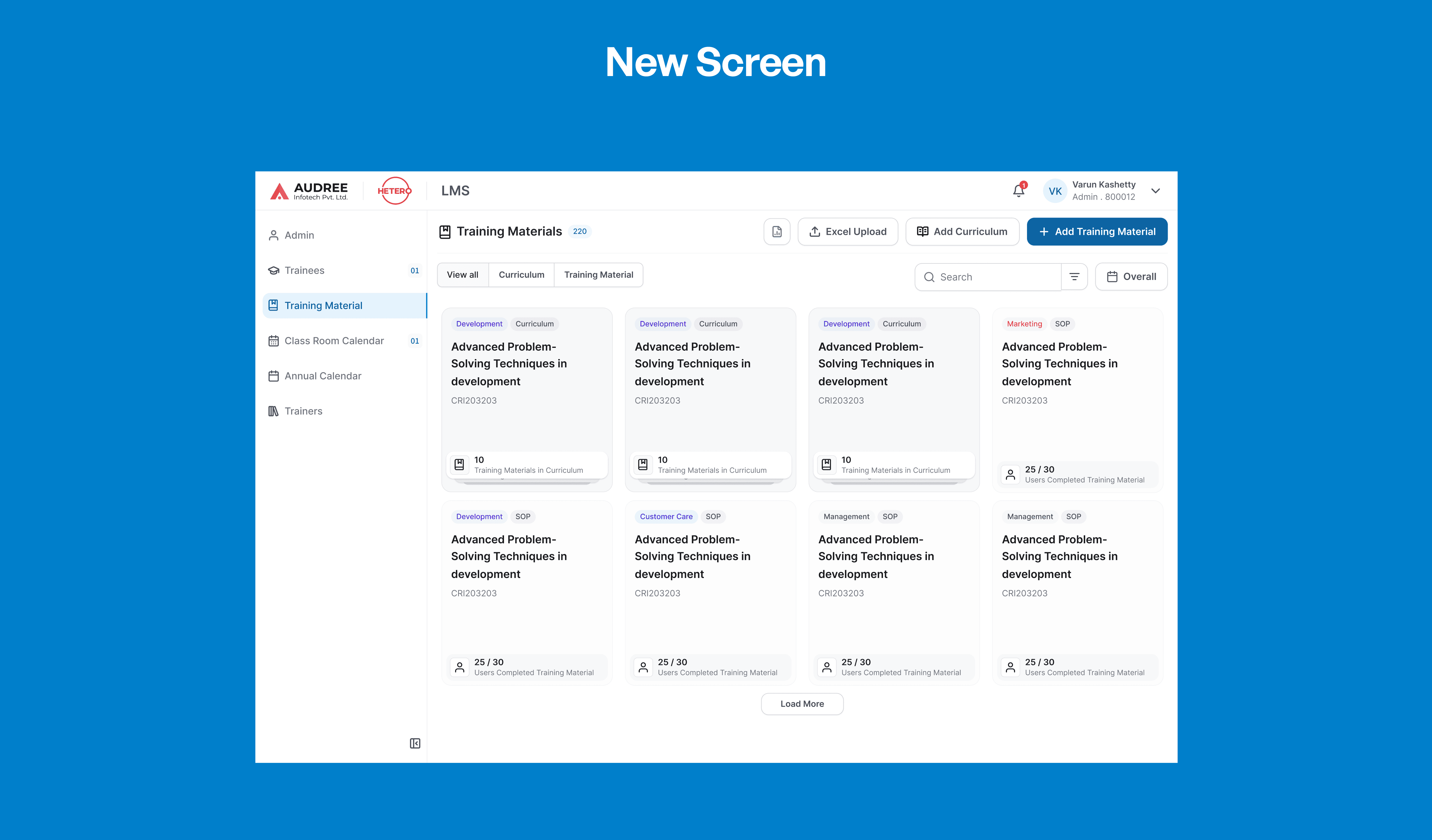
A Consistent UI for Faster, Confident Execution
A Consistent UI for Faster, Confident Execution
A Consistent UI for Faster, Confident Execution
A consistent UI language across screens helps teams work faster and with fewer errors. Standardised components and clear cues guide users smoothly through tasks, reducing confusion.
A consistent UI language across screens helps teams work faster and with fewer errors. Standardised components and clear cues guide users smoothly through tasks, reducing confusion.



Results That Redefined LMS Training & Management
Results That Redefined LMS Training & Management
Results That Redefined LMS Training & Management
Clear training workflows, centralised visibility, and guided reviews transformed how organisations plan, track, and validate employee training across departments.
Clear training workflows, centralised visibility, and guided reviews transformed how organisations plan, track, and validate employee training across departments.



Stronger Compliance & Traceability
Stronger Compliance & Traceability
Stronger Compliance & Traceability
Centralized training records and role-based approvals ensured all trainings were traceable, audit-ready, and free from manual follow-ups.
Centralized training records and role-based approvals ensured all trainings were traceable, audit-ready, and free from manual follow-ups.
Centralized training records and role-based approvals ensured all trainings were traceable, audit-ready, and free from manual follow-ups.

Faster API Batch Execution
Clear trainee lists, status indicators, and guided reviewer actions enabled managers to identify pending trainings, validate completions, and close reviews faster without emails, spreadsheets, or follow-ups.

Fewer Support Tickets
Unified screens and clear status visibility simplified training reviews, reduced confusion, and cut down repeated coordination and support queries.


Faster API Batch Execution
Faster API Batch Execution
Clear trainee lists, status indicators, and guided reviewer actions enabled managers to identify pending trainings, validate completions, and close reviews faster without emails, spreadsheets, or follow-ups.
Clear trainee lists, status indicators, and guided reviewer actions enabled managers to identify pending trainings, validate completions, and close reviews faster without emails, spreadsheets, or follow-ups.


Fewer Support Tickets
Fewer Support Tickets
Unified screens and clear status visibility simplified training reviews, reduced confusion, and cut down repeated coordination and support queries.
Unified screens and clear status visibility simplified training reviews, reduced confusion, and cut down repeated coordination and support queries.
Deep-Dive Into More System
Deep-Dive Into More System
Deep-Dive Into More System
Browse every optimised Software and explore how legacy systems became intuitive.
Browse every optimised Software and explore how legacy systems became intuitive.
Browse every optimised Software and explore how legacy systems became intuitive.
RCAI


Root Cause Analysis with Intelligence
LMS
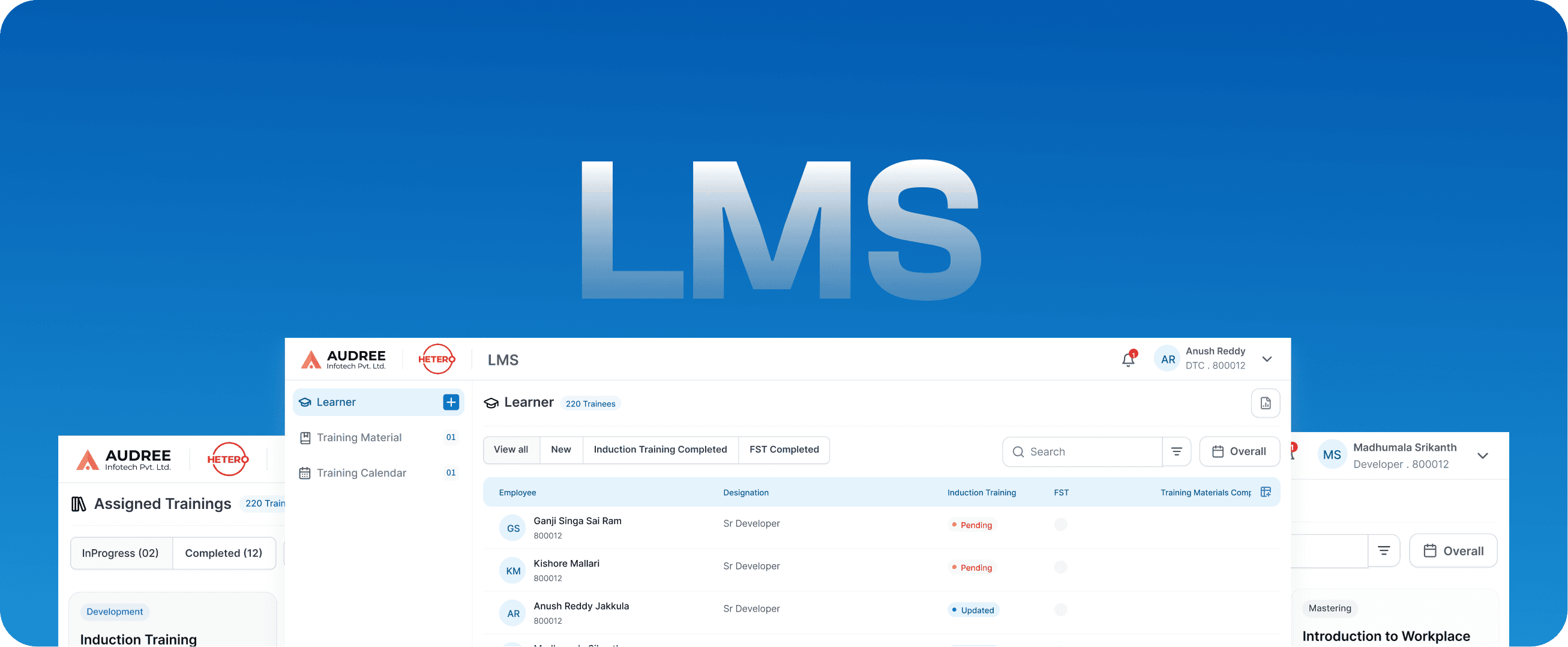

Learning Management System
LIMS
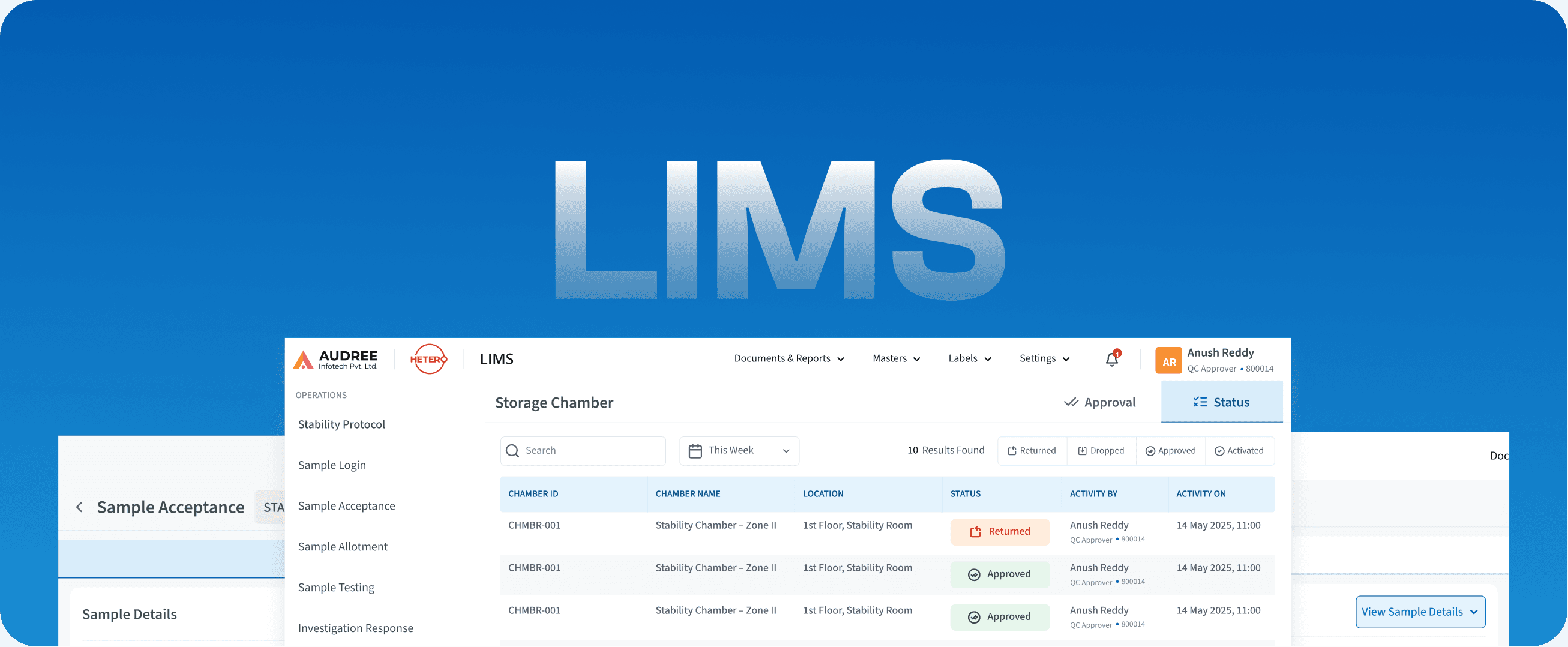

Laboratory Information Management System
S & OP
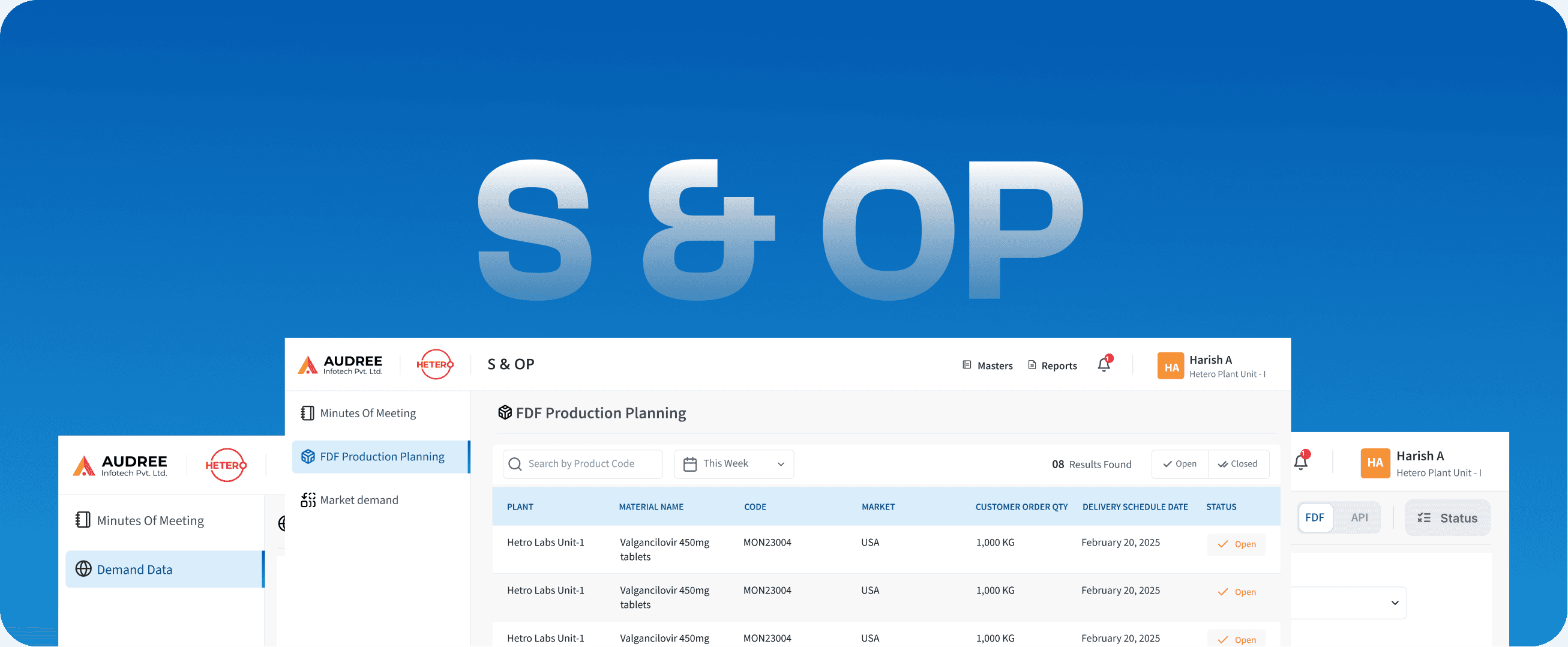

Sales & Operations Planning
E-BMR
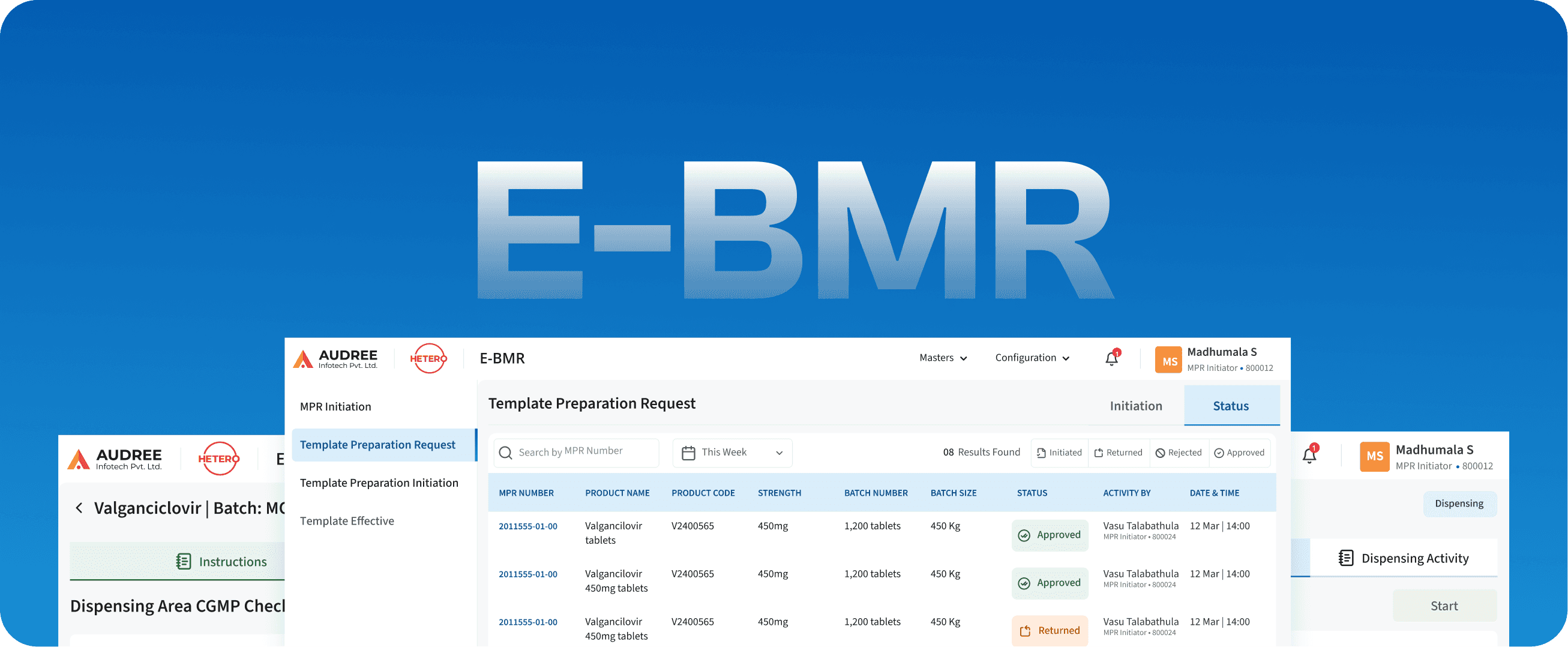

Batch Manufacturing Recall
WMPS


Warehouse Management System
DMS
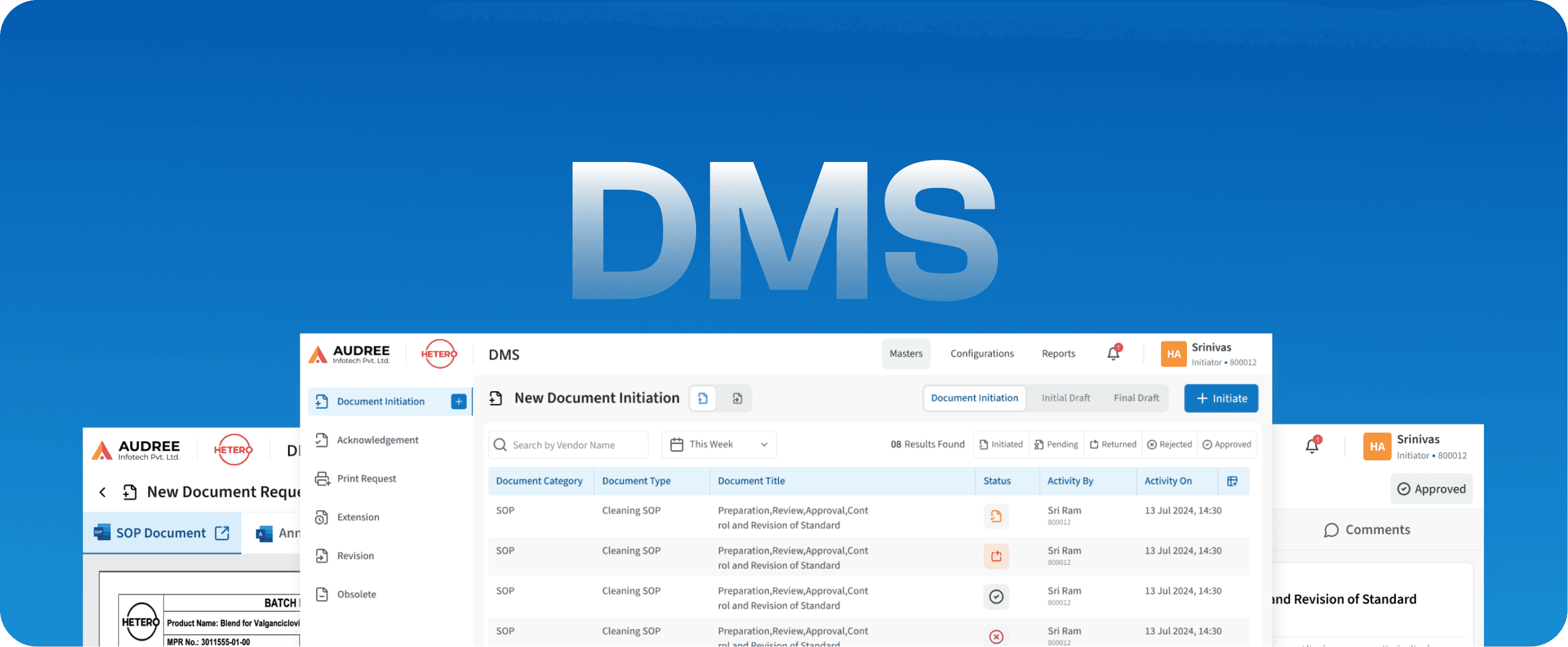

Document Management System
CAPA


Corrective And Preventive Actions
QAS


Quality Agreement System
Vendor Portal
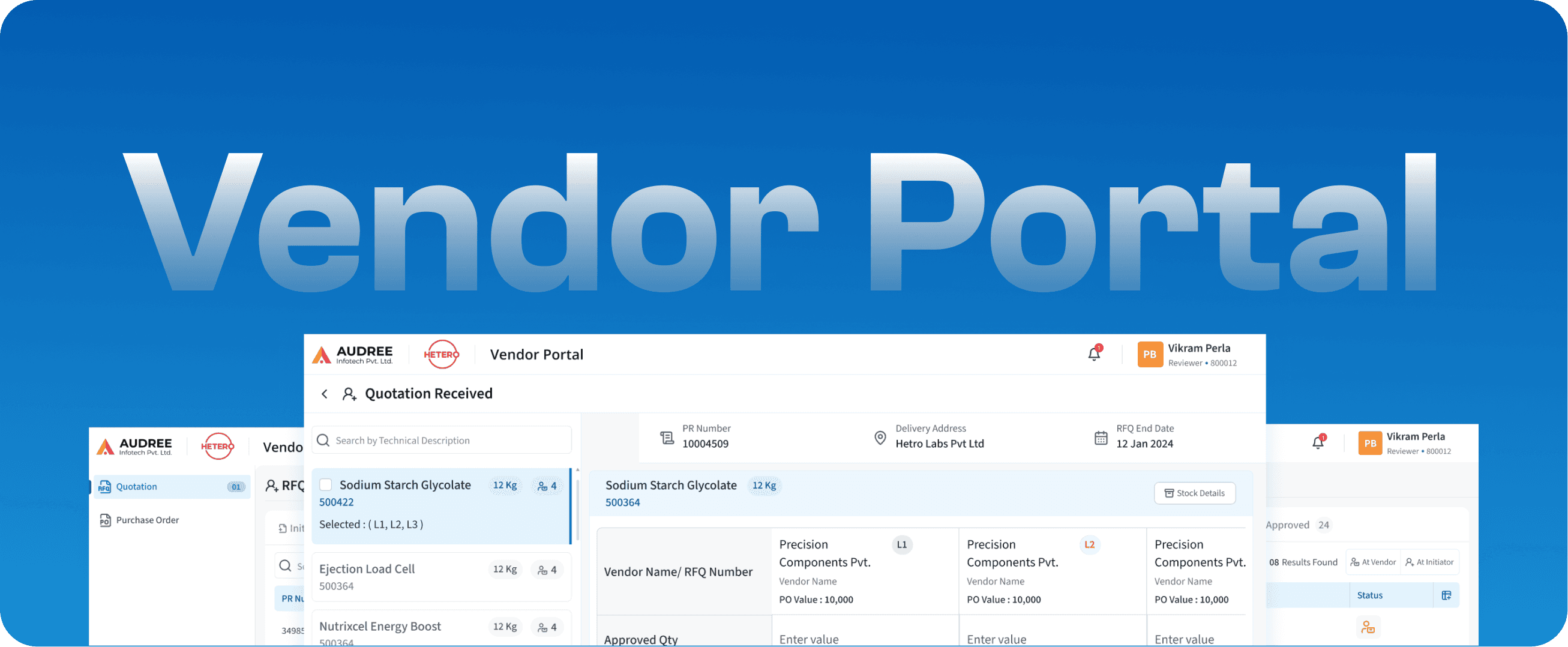

Vendor Management System
LIR-AER
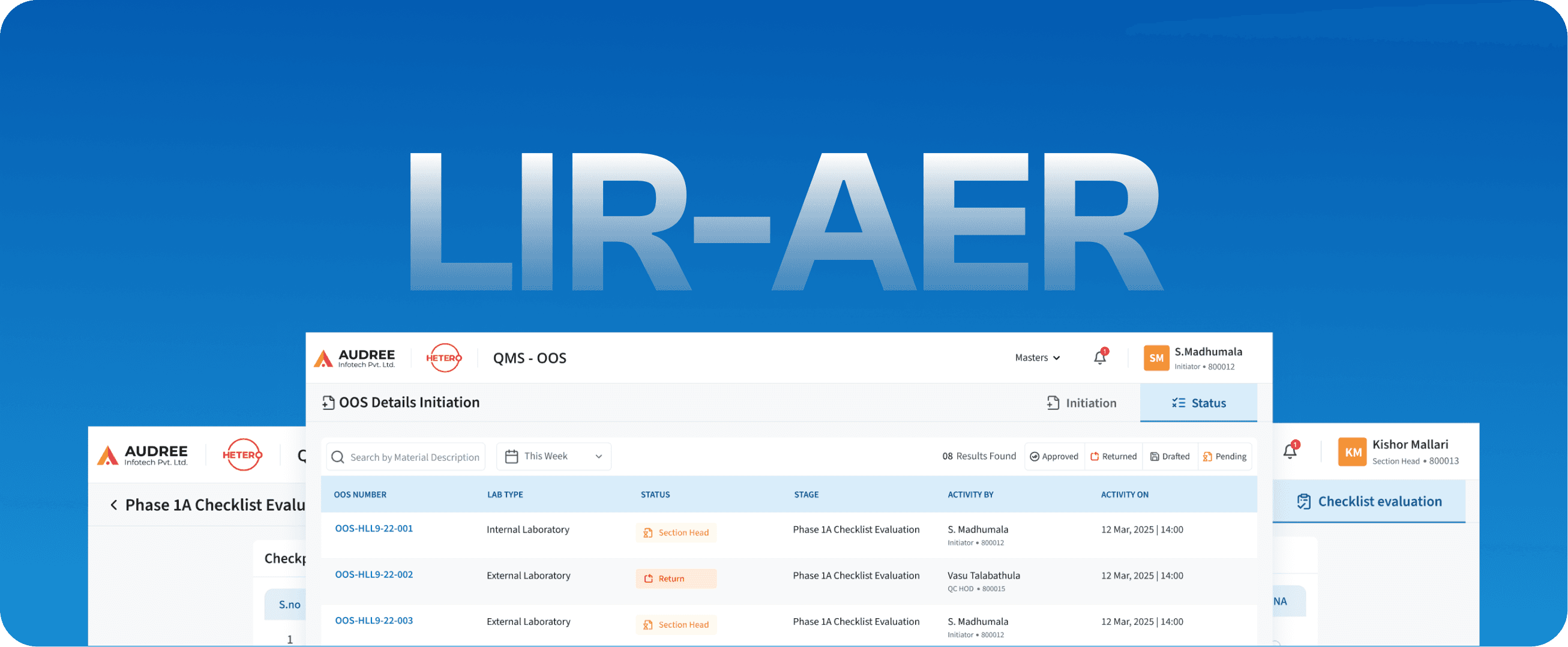

Laboratory Information Record
OOS
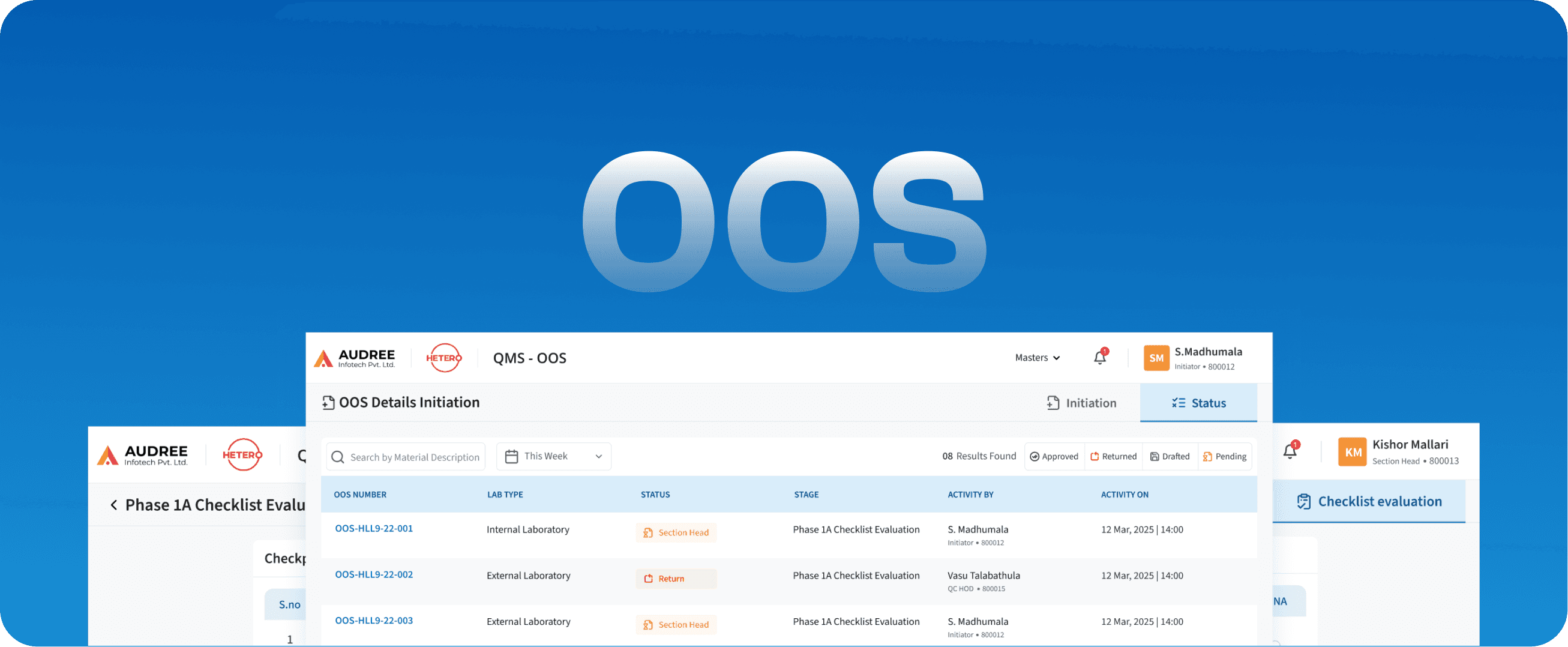

Out Of Specification
CMS
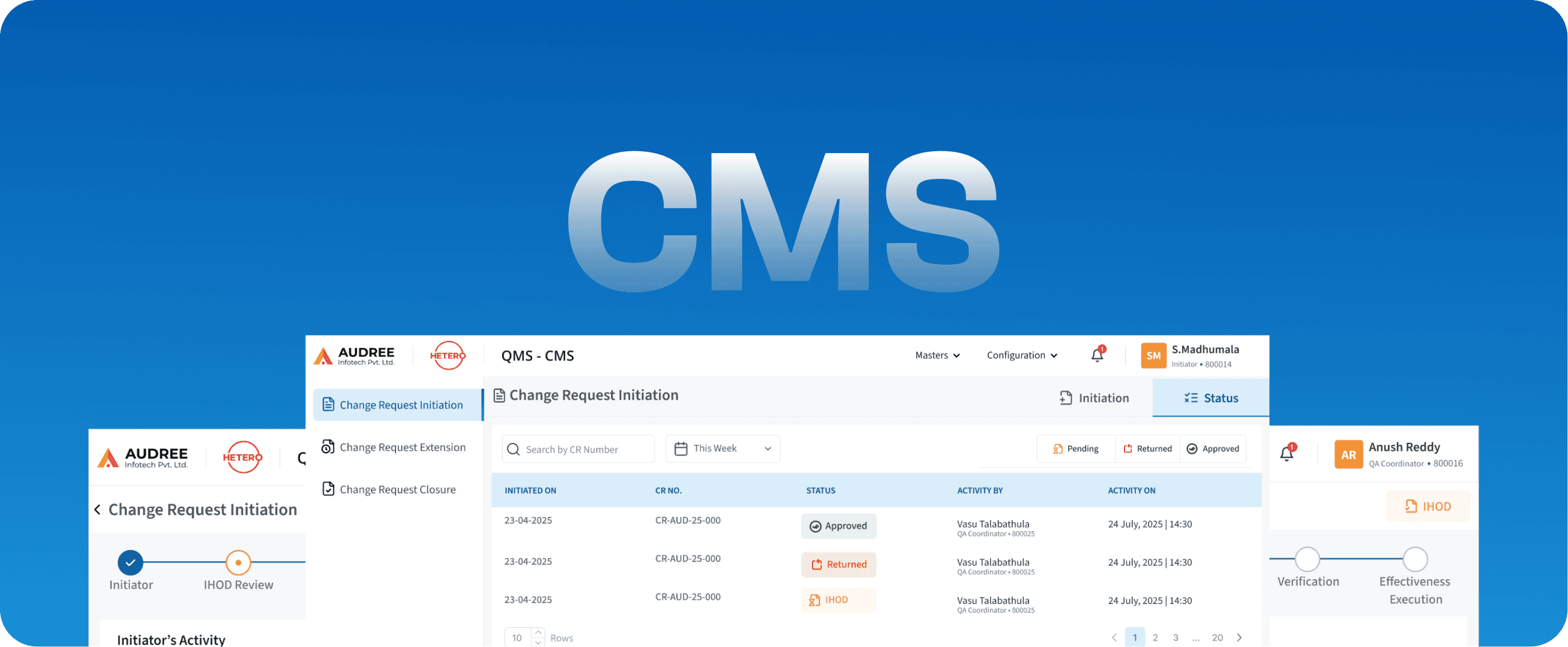

Change Management System
IMS
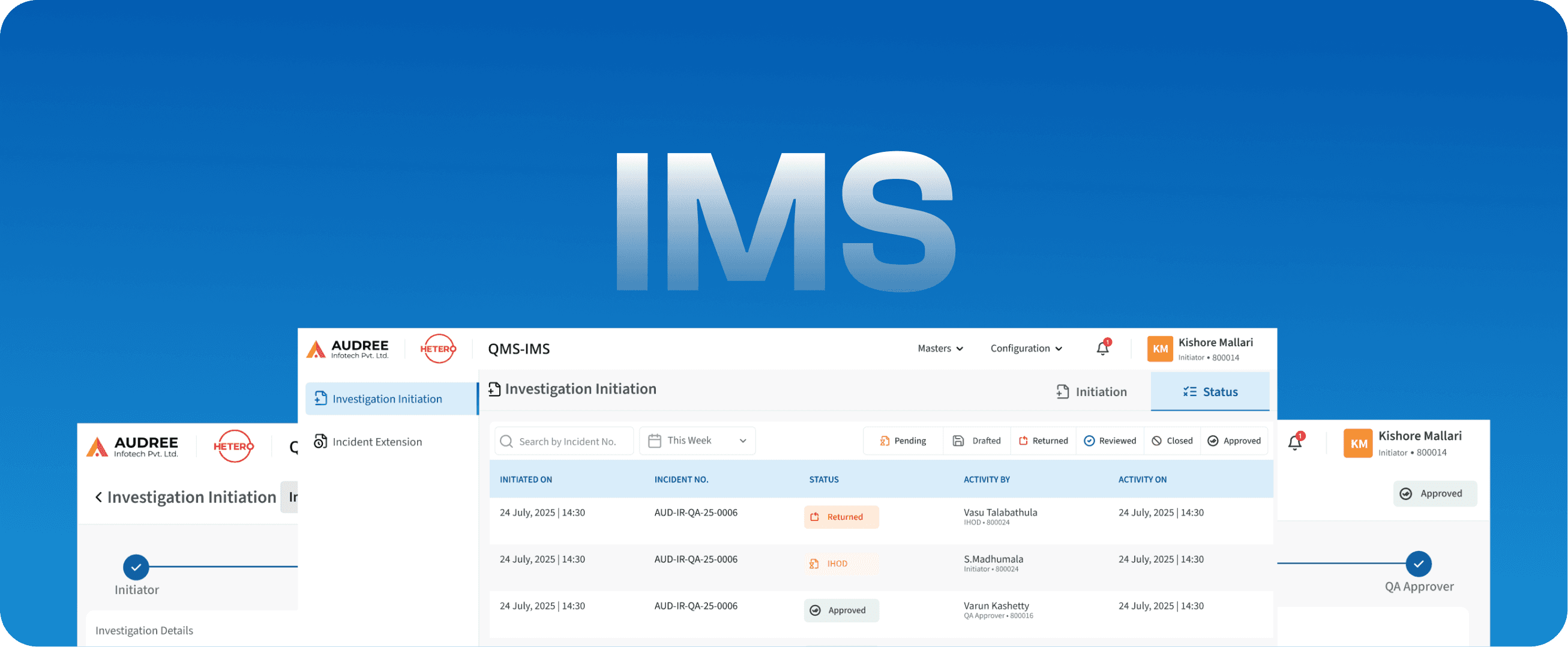

Incident Management System
BRMS-API
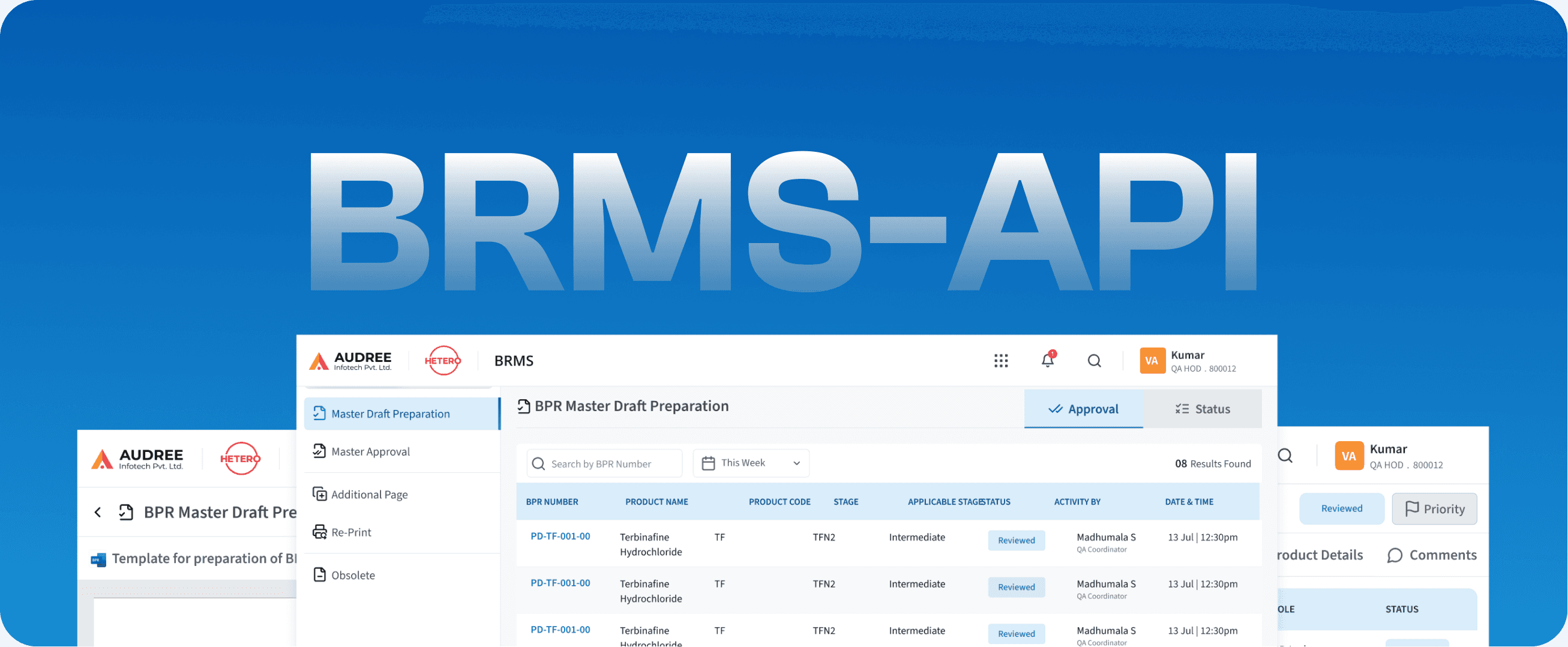

Batch Record- Active Pharmaceutical Ingredient
E-BRMS
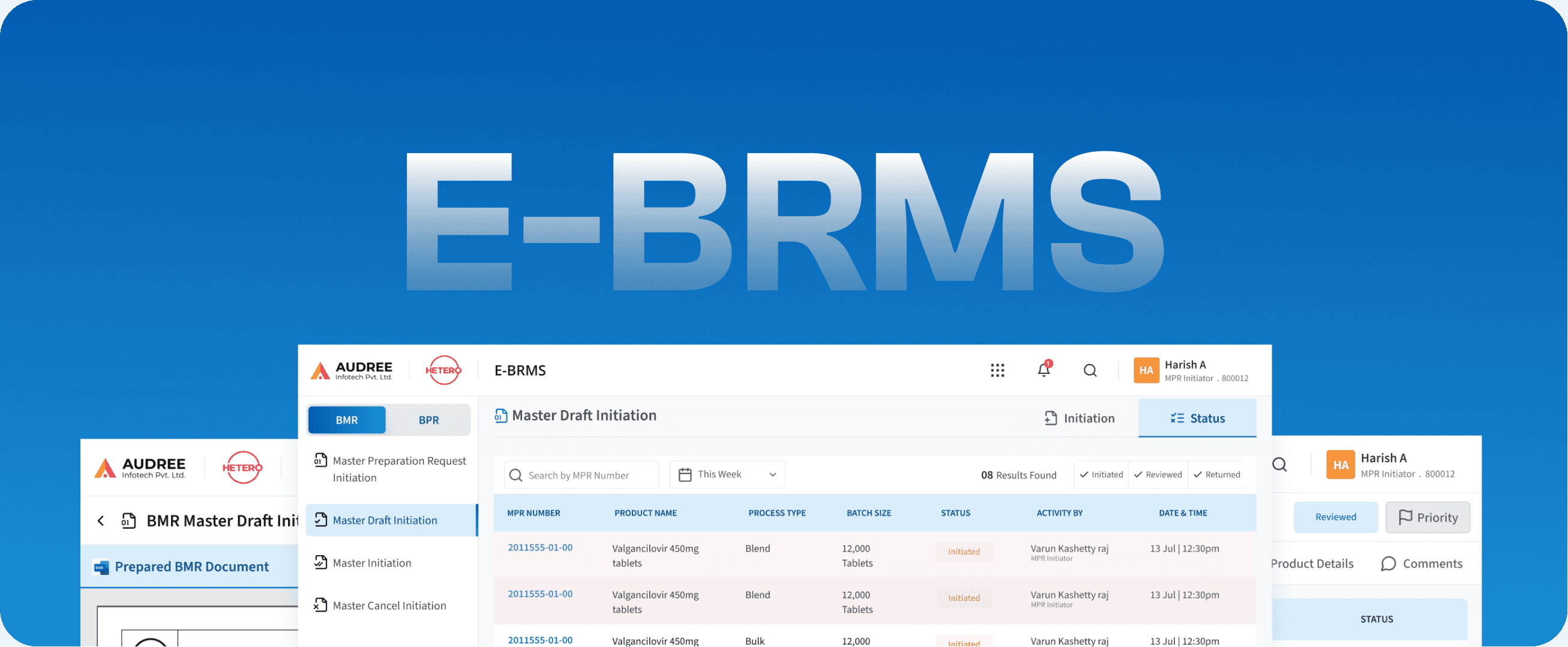

Batch Record Management System
RIMS
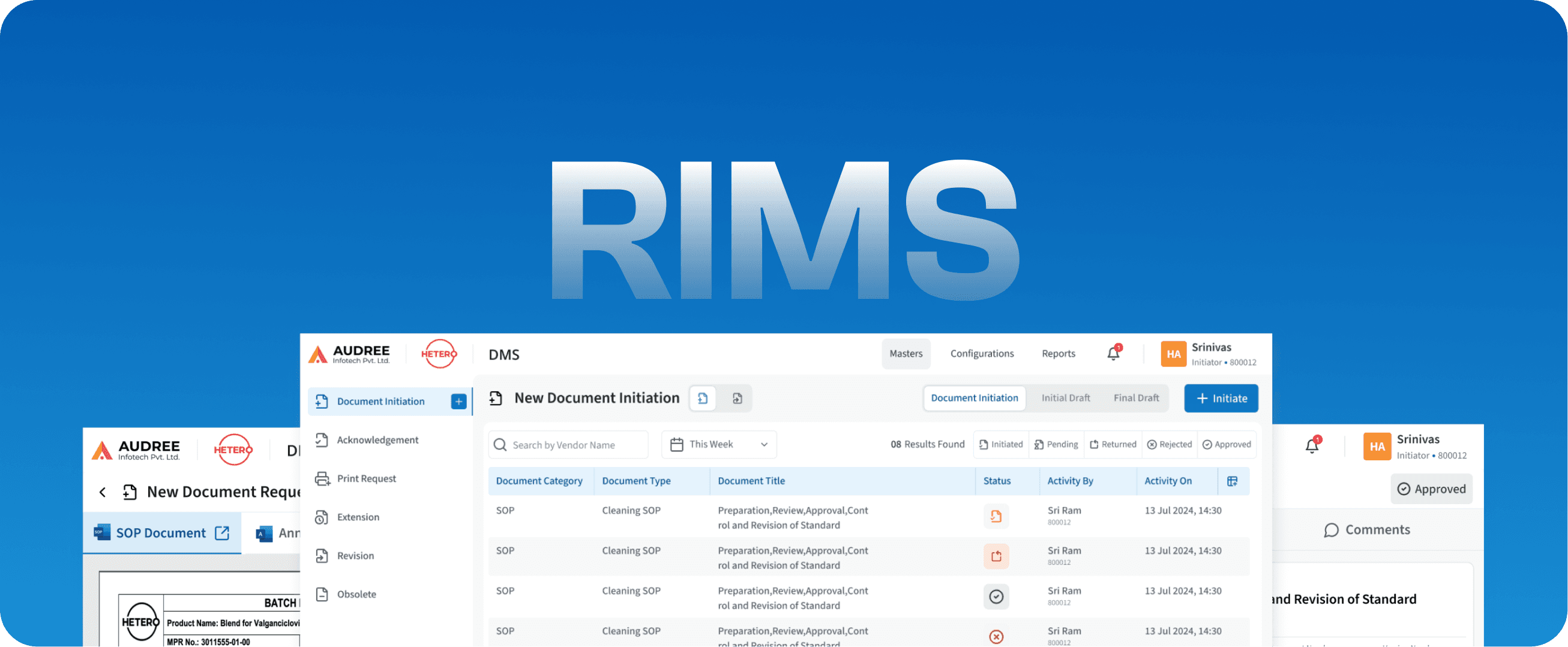

Regulatory Information Management System
APQR
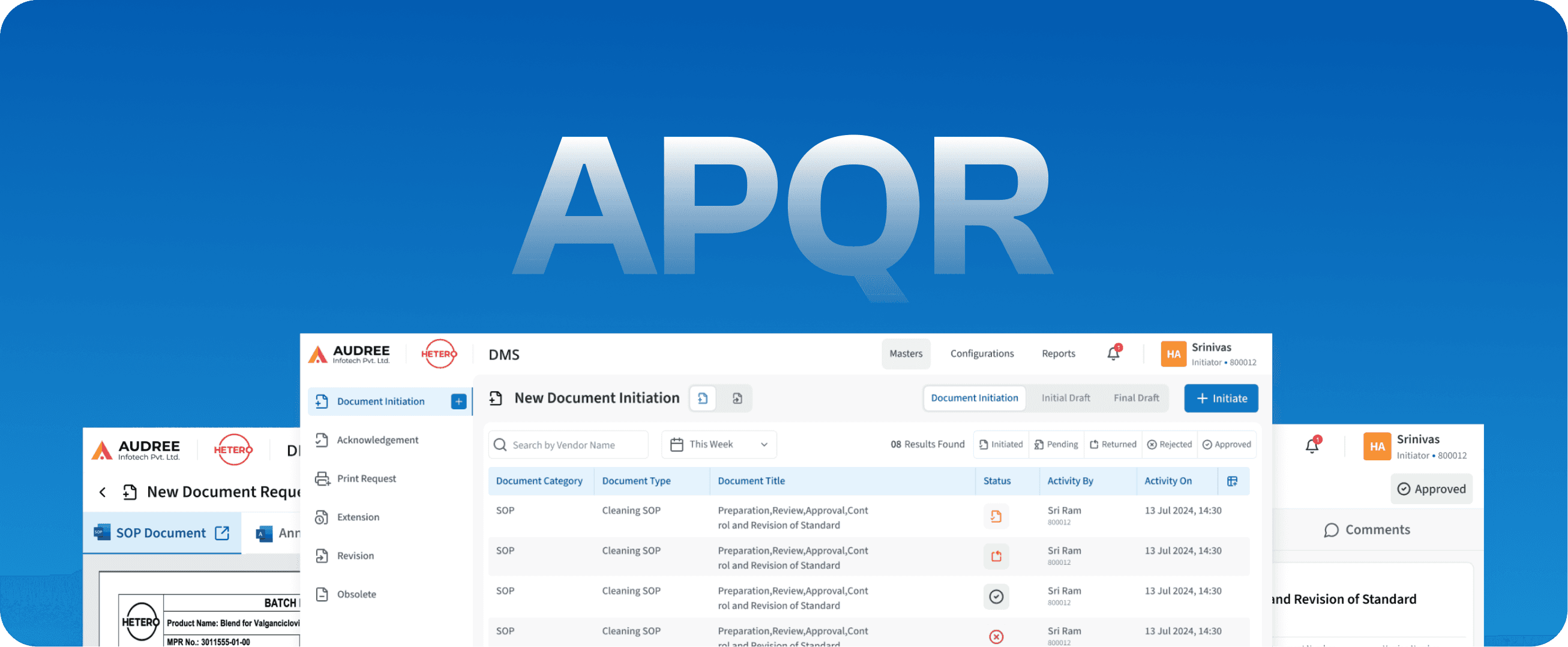

Annual Product Quality Review
RCAI

Root Cause Analysis with Intelligence
LMS

Learning Management System
LIMS

Laboratory Information Management System
S & OP

Sales & Operations Planning
E-BMR

Batch Manufacturing Recall
RIMS
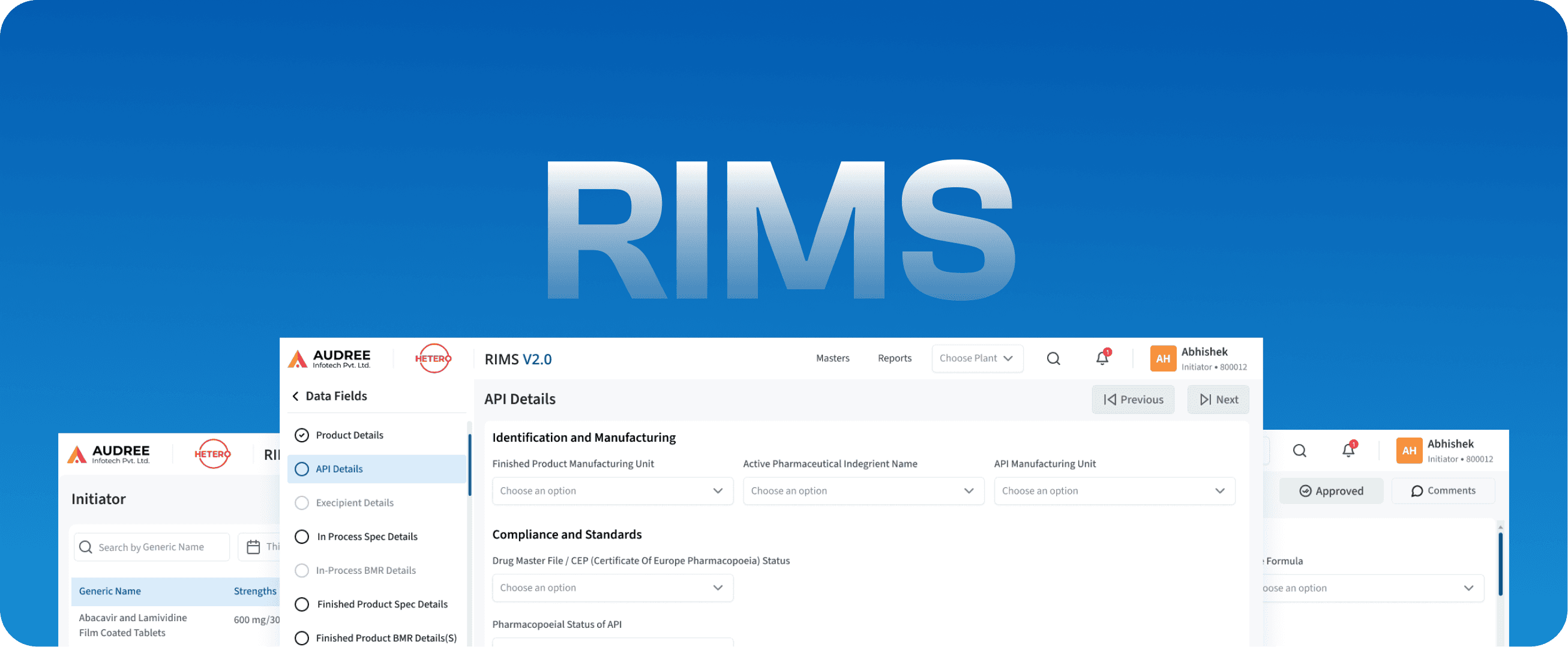
Regulatory Information Management Systems
IMS

Incident Management System
BRMS-API

Batch Record- Active Pharmaceutical Ingredient
E-BRMS

Batch Record Management System
APQR

Annual Product Quality Review
RIMS

Regulatory Information Management System
WMPS

Warehouse Management System
DMS

Document Management System
CMS

Change Management System
OOS

Out Of Specification
LIR-AER

Laboratory Information Record
Vendor Portal

Vendor Management System
QAS

Quality Agreement System
CAPA

Corrective And Preventive Actions

