Designing a Smarter Way to Identify Root Causes with Intelligence Support.
Designing a Smarter Way to Identify Root Causes with Intelligence Support.
Designing a Smarter Way to Identify Root Causes with Intelligence Support.
About Project
About Project
About Project
Root Cause Analysis with Intelligence (RCAI) is a concept-led initiative created to improve how pharmaceutical teams identify and document root causes during investigations. Audree observed that traditional RCA depended heavily on individual experience, varied widely between users, and often repeated the same analysis without learning from past cases.
The goal of RCAI was to design a clear, guided investigation flow from scratch. We mapped the journey from incident initiation to root cause confirmation and CAPA alignment, embedding intelligence where it adds value. This approach helps teams work consistently, reuse past learnings, and produce audit-ready investigations without increasing complexity.
Root Cause Analysis with Intelligence (RCAI) is a concept-led initiative created to improve how pharmaceutical teams identify and document root causes during investigations. Audree observed that traditional RCA depended heavily on individual experience, varied widely between users, and often repeated the same analysis without learning from past cases.
The goal of RCAI was to design a clear, guided investigation flow from scratch. We mapped the journey from incident initiation to root cause confirmation and CAPA alignment, embedding intelligence where it adds value. This approach helps teams work consistently, reuse past learnings, and produce audit-ready investigations without increasing complexity.
About Project
Pharmaceutical
Team
Varun , S.Madhumala
Subscription Category
Quick win
Project start Year
August 2024
Core Business Challenges
Core Business Challenges
Core Business Challenges
Repeated Investigations With Limited Learning
Repeated Investigations With Limited Learning
Repeated Investigations With Limited Learning
Similar issues were repeatedly investigated because individual learnings were not captured or shared across investigations.
Similar issues were repeatedly investigated because individual learnings were not captured or shared across investigations.
Similar issues were repeatedly investigated because individual learnings were not captured or shared across investigations.
Inconsistent Root Cause Outcomes
Inconsistent Root Cause Outcomes
Inconsistent Root Cause Outcomes
Root cause analysis varied across teams and sites, leading to uneven investigation quality and difficulty in defending outcomes during audits.
Root cause analysis varied across teams and sites, leading to uneven investigation quality and difficulty in defending outcomes during audits.
Root cause analysis varied across teams and sites, leading to uneven investigation quality and difficulty in defending outcomes during audits.
Heavy Dependence on Manual Coordination
Heavy Dependence on Manual Coordination
Investigations required frequent follow ups between QA, QC, and other teams, causing delays and loss of investigation context.
Investigations required frequent follow ups between QA, QC, and other teams, causing delays and loss of investigation context.
Heavy Dependence on Manual Coordination
Investigations required frequent follow ups between QA, QC, and other teams, causing delays and loss of investigation context.
Our Approach
Our Approach
Our Approach
Mapping the RCAI Investigation Flow
We mapped the RCAI investigation flow from incident initiation to root cause confirmation, introducing guided questions and intelligence led cause suggestions to reduce guesswork. The flow allows users to validate, regenerate, or define causes, ensuring investigations reuse past learnings and continuously improve over time.
Mapping the RCAI Investigation Flow
We mapped the RCAI investigation flow from incident initiation to root cause confirmation, introducing guided questions and intelligence led cause suggestions to reduce guesswork. The flow allows users to validate, regenerate, or define causes, ensuring investigations reuse past learnings and continuously improve over time.
Mapping the RCAI Investigation Flow
We mapped the RCAI investigation flow from incident initiation to root cause confirmation, introducing guided questions and intelligence led cause suggestions to reduce guesswork. The flow allows users to validate, regenerate, or define causes, ensuring investigations reuse past learnings and continuously improve over time.
Designing RCAI Into a Guided Investigation Workflow
Designing RCAI Into a Guided Investigation Workflow
Designing RCAI Into a Guided Investigation Workflow
The RCAI process was designed as a single, guided investigation workflow with clear stages from incident initiation to root cause confirmation and CAPA alignment. Structured reasoning and contextual intelligence reduced manual coordination, improved consistency, and strengthened audit readiness across teams.
The RCAI process was designed as a single, guided investigation workflow with clear stages from incident initiation to root cause confirmation and CAPA alignment. Structured reasoning and contextual intelligence reduced manual coordination, improved consistency, and strengthened audit readiness across teams.
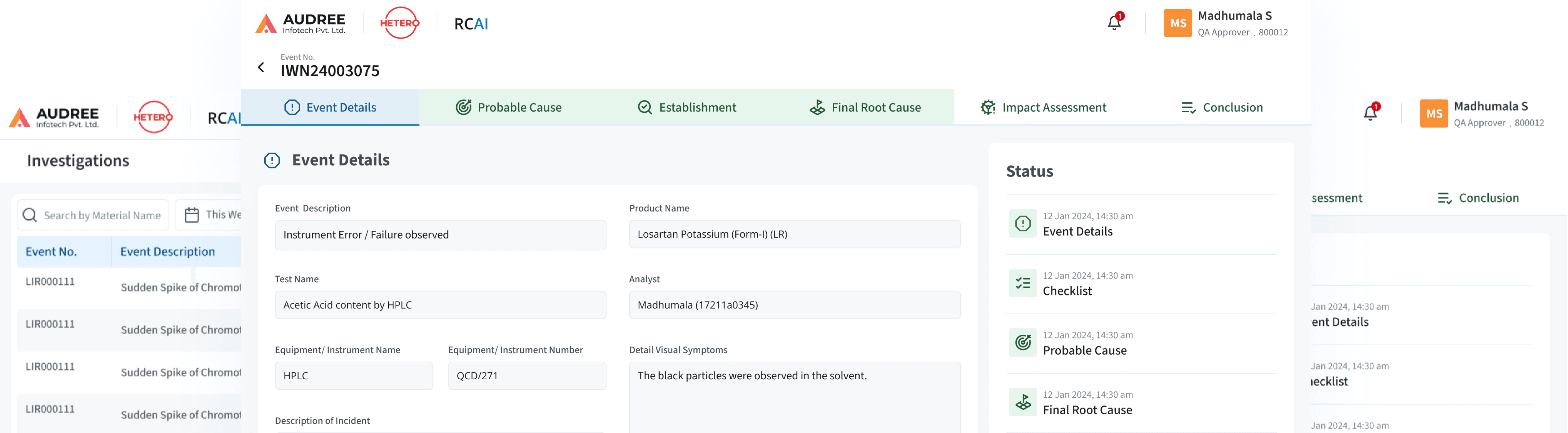





Structured Workflow for Root Cause Identification
Structured Workflow for Root Cause Identification
Structured Workflow for Root Cause Identification
The RCAI workflow became a clear, step-by-step investigation flow from incident initiation to root cause confirmation, removing reliance on fragmented discussions and manual reasoning.
Each team can clearly see:
Investigation context and responses
Suggested and confirmed root causes
Pending validations and next steps
This turns root cause analysis from an experience-driven exercise into a guided, consistent, and defensible investigation workflow.
The RCAI workflow became a clear, step-by-step investigation flow from incident initiation to root cause confirmation, removing reliance on fragmented discussions and manual reasoning.
Each team can clearly see:
Investigation context and responses
Suggested and confirmed root causes
Pending validations and next steps
This turns root cause analysis from an experience-driven exercise into a guided, consistent, and defensible investigation workflow.
The RCAI workflow became a clear, step-by-step investigation flow from incident initiation to root cause confirmation, removing reliance on fragmented discussions and manual reasoning.
Each team can clearly see:
Investigation context and responses
Suggested and confirmed root causes
Pending validations and next steps
This turns root cause analysis from an experience-driven exercise into a guided, consistent, and defensible investigation workflow.
Intelligent Root Cause Suggestions
Intelligent Root Cause Suggestions
Intelligent Root Cause Suggestions
RCAI uses investigation inputs and historical learnings to suggest relevant probable root causes early in the process. Investigators can validate, refine, or regenerate suggestions instead of starting from a blank slate.
This helps teams reduce guesswork, reuse past insights, and arrive at accurate, defensible root causes faster.
RCAI uses investigation inputs and historical learnings to suggest relevant probable root causes early in the process. Investigators can validate, refine, or regenerate suggestions instead of starting from a blank slate.
This helps teams reduce guesswork, reuse past insights, and arrive at accurate, defensible root causes faster.
RCAI uses investigation inputs and historical learnings to suggest relevant probable root causes early in the process. Investigators can validate, refine, or regenerate suggestions instead of starting from a blank slate.
This helps teams reduce guesswork, reuse past insights, and arrive at accurate, defensible root causes faster.
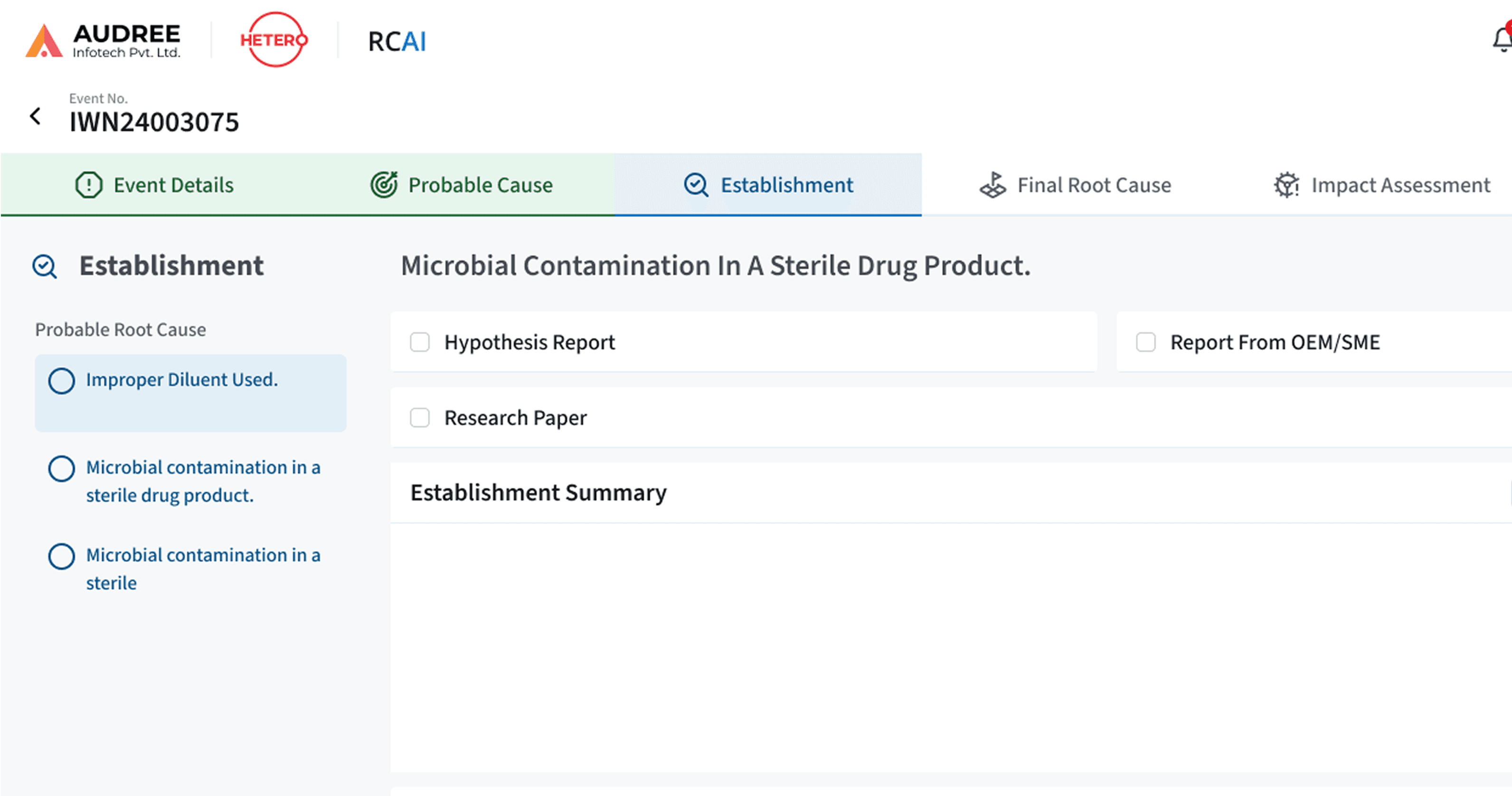


Clear Visual Guidance for Root Cause Identification
Clear Visual Guidance for Root Cause Identification
Clear Visual Guidance for Root Cause Identification
Clear investigation stages, cause indicators, and validation states guide users through RCAI, making progress and next steps easy to understand.
Clear investigation stages, cause indicators, and validation states guide users through RCAI, making progress and next steps easy to understand.
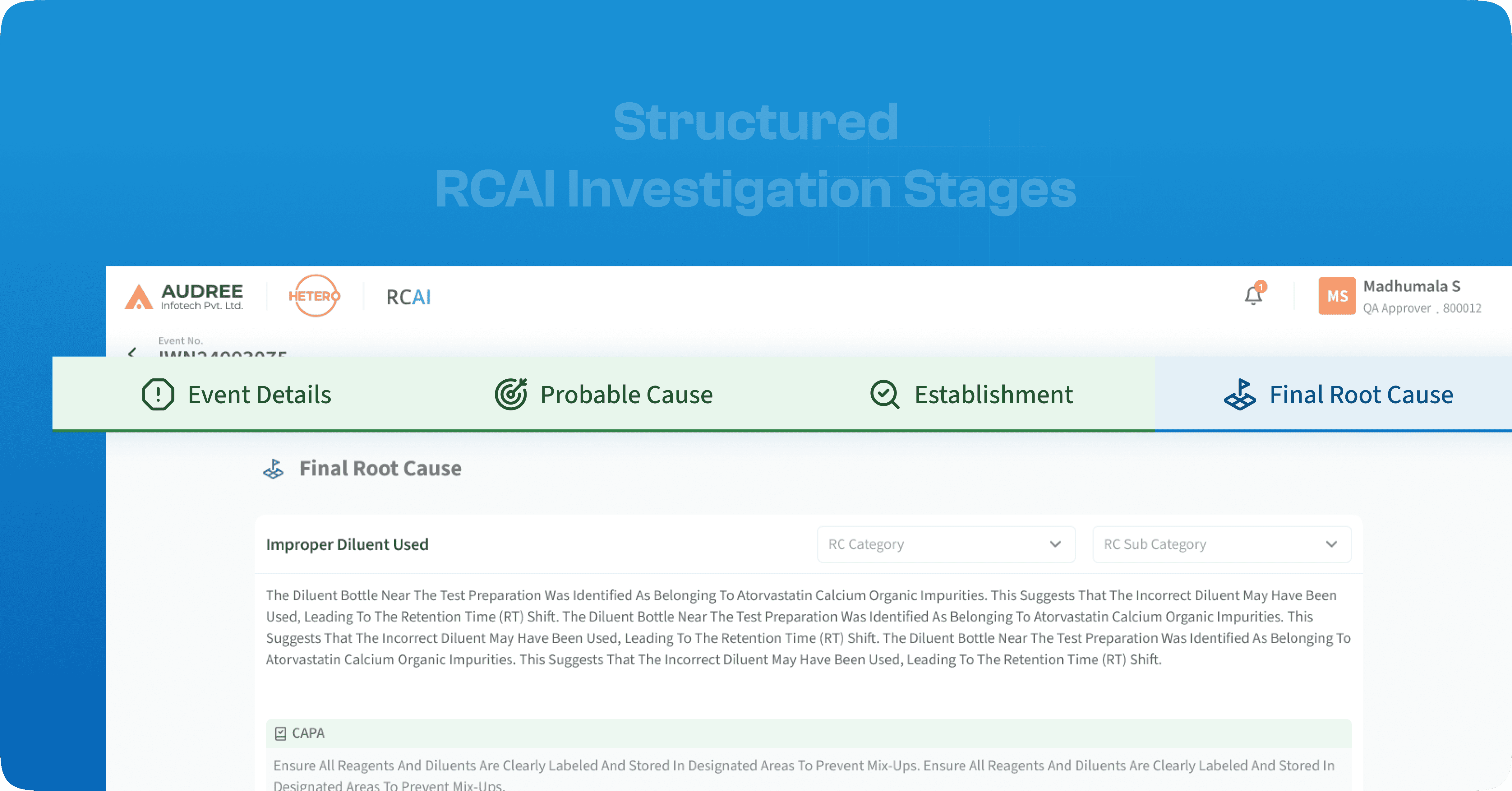



Structured UI for Smarter, Root Cause Identification
Structured UI for Smarter, Root Cause Identification
Structured UI for Smarter, Root Cause Identification
The RCAI UI guides each investigation stage with clear visuals and cues, reducing errors and keeping teams aligned throughout the analysis.
The RCAI UI guides each investigation stage with clear visuals and cues, reducing errors and keeping teams aligned throughout the analysis.



Results That Introduced Investigation Lifecycle
Results That Introduced Investigation Lifecycle
Results That Introduced Investigation Lifecycle
Clearer investigation flows, connected RCAI stages, and structured reasoning steps improved investigation speed, reduced errors, and strengthened confidence in root cause decisions.
Clearer investigation flows, connected RCAI stages, and structured reasoning steps improved investigation speed, reduced errors, and strengthened confidence in root cause decisions.



Improved Root Cause Clarity
Improved Root Cause Clarity
Improved Root Cause Clarity
Structured investigation stages made it easier for teams to understand, review, and validate root causes, reducing confusion and rework across investigations.
Structured investigation stages made it easier for teams to understand, review, and validate root causes, reducing confusion and rework across investigations.
Structured investigation stages made it easier for teams to understand, review, and validate root causes, reducing confusion and rework across investigations.

Faster, More Effective Investigations
Intelligent root cause suggestions and clear progression replaced manual back-and-forth, helping teams reach accurate conclusions faster with fewer rework cycles.

Reduced Investigation Repetition
Reuse of past investigation learnings prevented teams from re-analyzing similar quality events repeatedly, saving time and improving overall investigation efficiency.


Faster, More Effective Investigations
Faster, More Effective Investigations
Intelligent root cause suggestions and clear progression replaced manual back-and-forth, helping teams reach accurate conclusions faster with fewer rework cycles.
Intelligent root cause suggestions and clear progression replaced manual back-and-forth, helping teams reach accurate conclusions faster with fewer rework cycles.


Reduced Investigation Repetition
Reduced Investigation Repetition
Reuse of past investigation learnings prevented teams from re-analyzing similar quality events repeatedly, saving time and improving overall investigation efficiency.
Reuse of past investigation learnings prevented teams from re-analyzing similar quality events repeatedly, saving time and improving overall investigation efficiency.
Deep-Dive Into More System
Deep-Dive Into More System
Deep-Dive Into More System
Browse every optimised Software and explore how legacy systems became intuitive.
Browse every optimised Software and explore how legacy systems became intuitive.
Browse every optimised Software and explore how legacy systems became intuitive.
RCAI


Root Cause Analysis with Intelligence
LMS
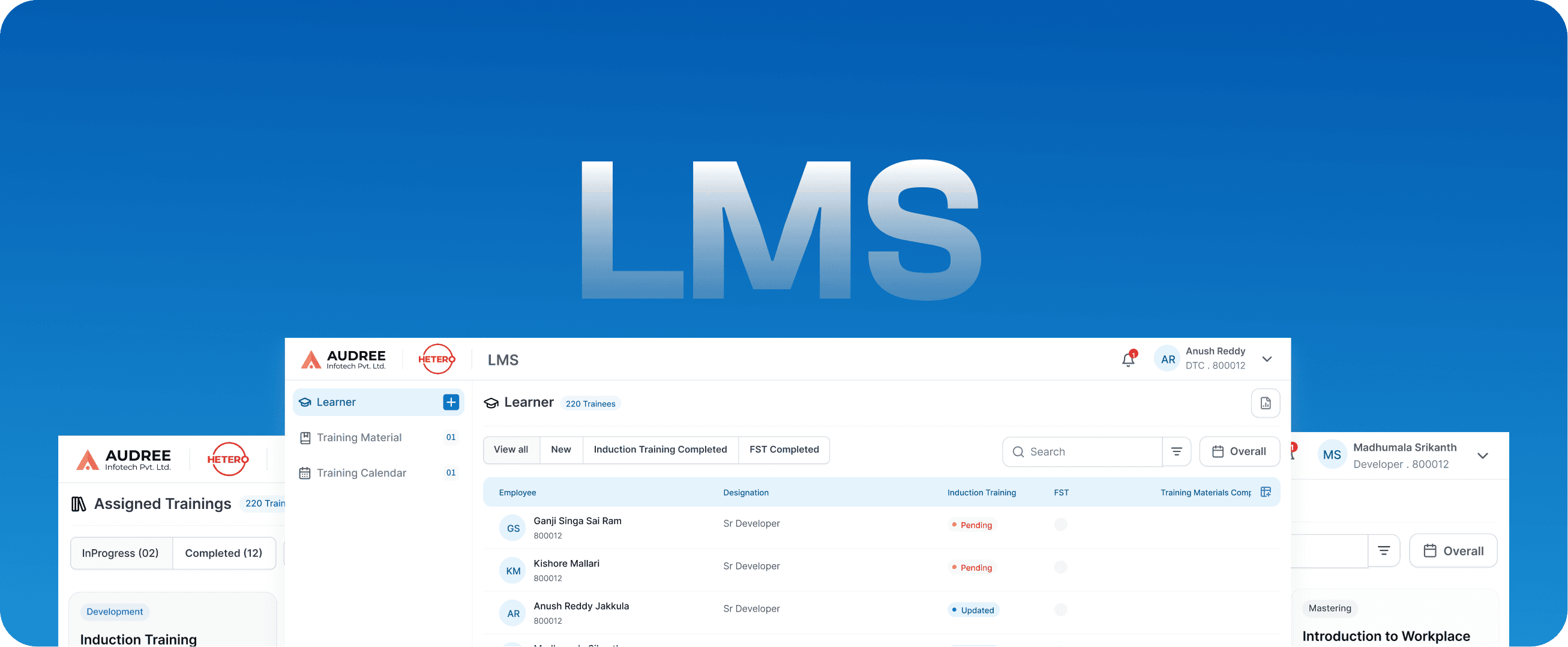

Learning Management System
LIMS
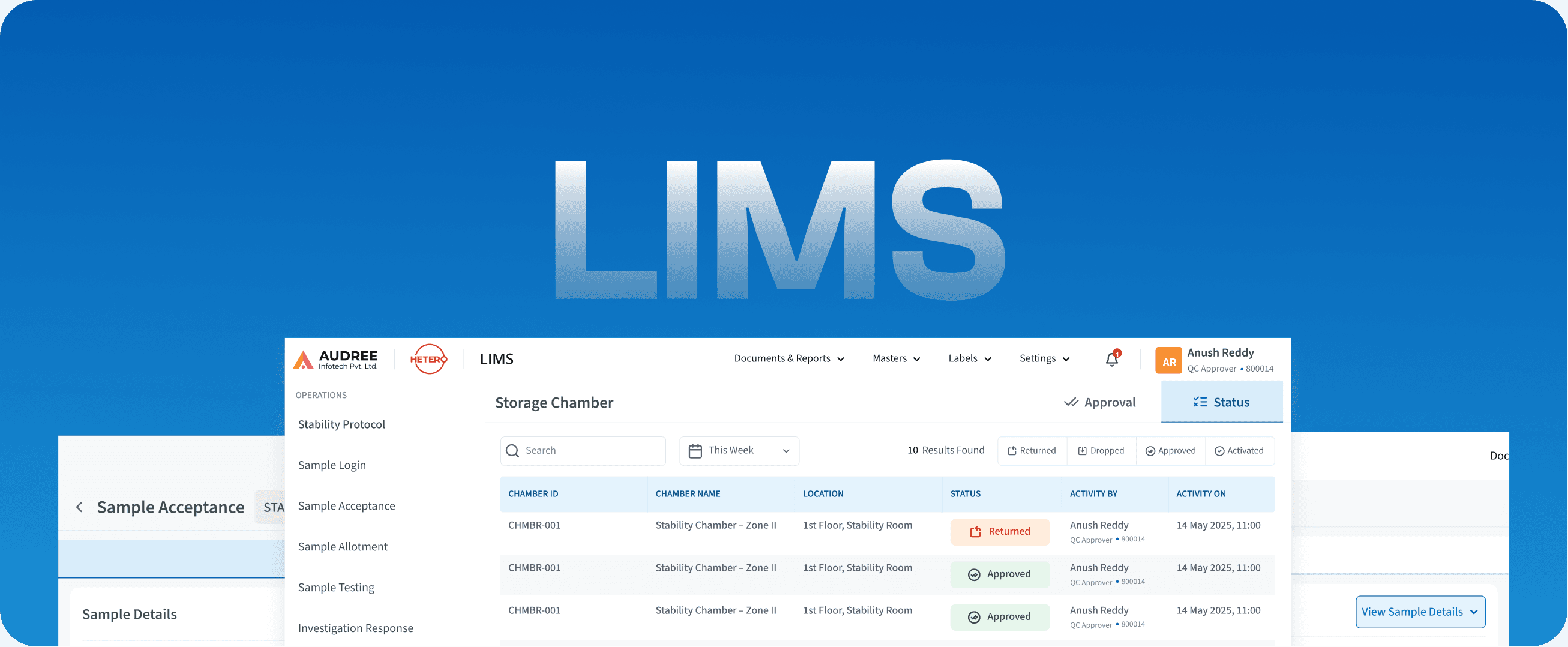

Laboratory Information Management System
S & OP
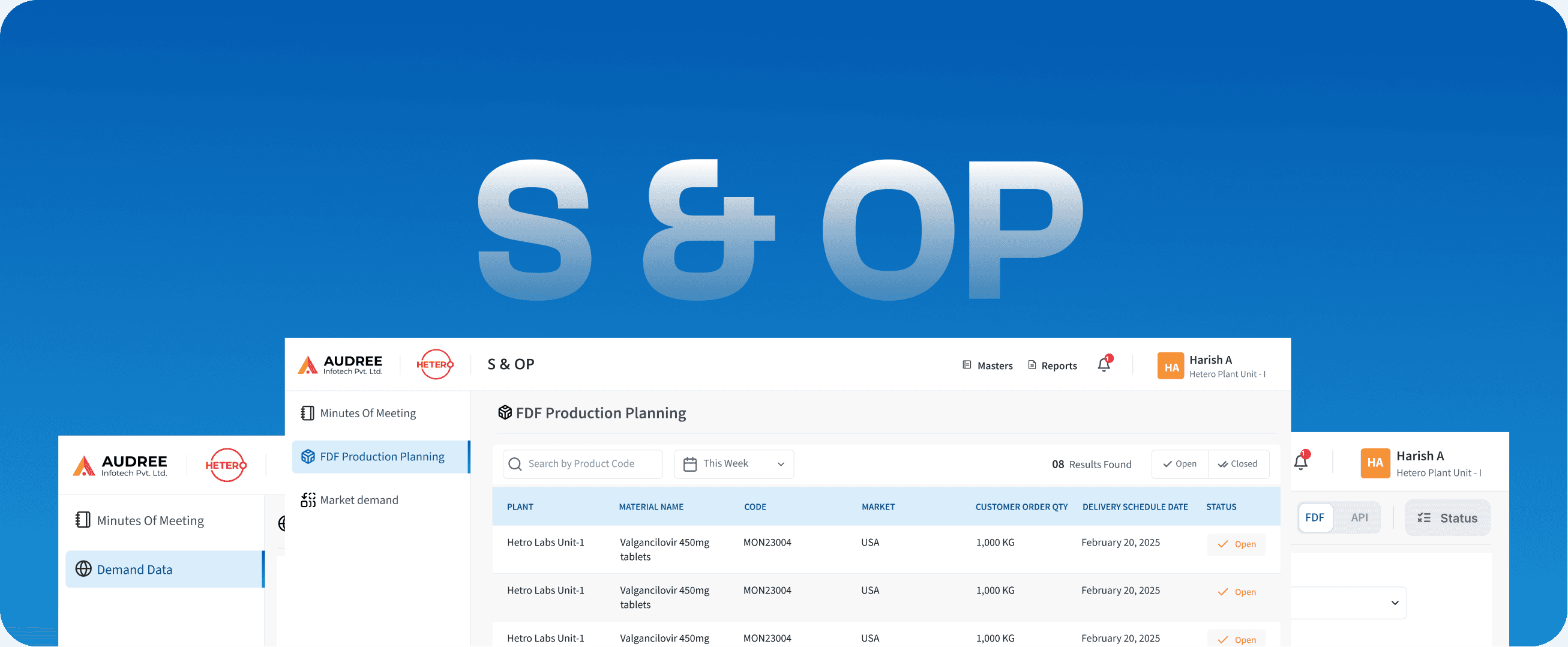

Sales & Operations Planning
E-BMR
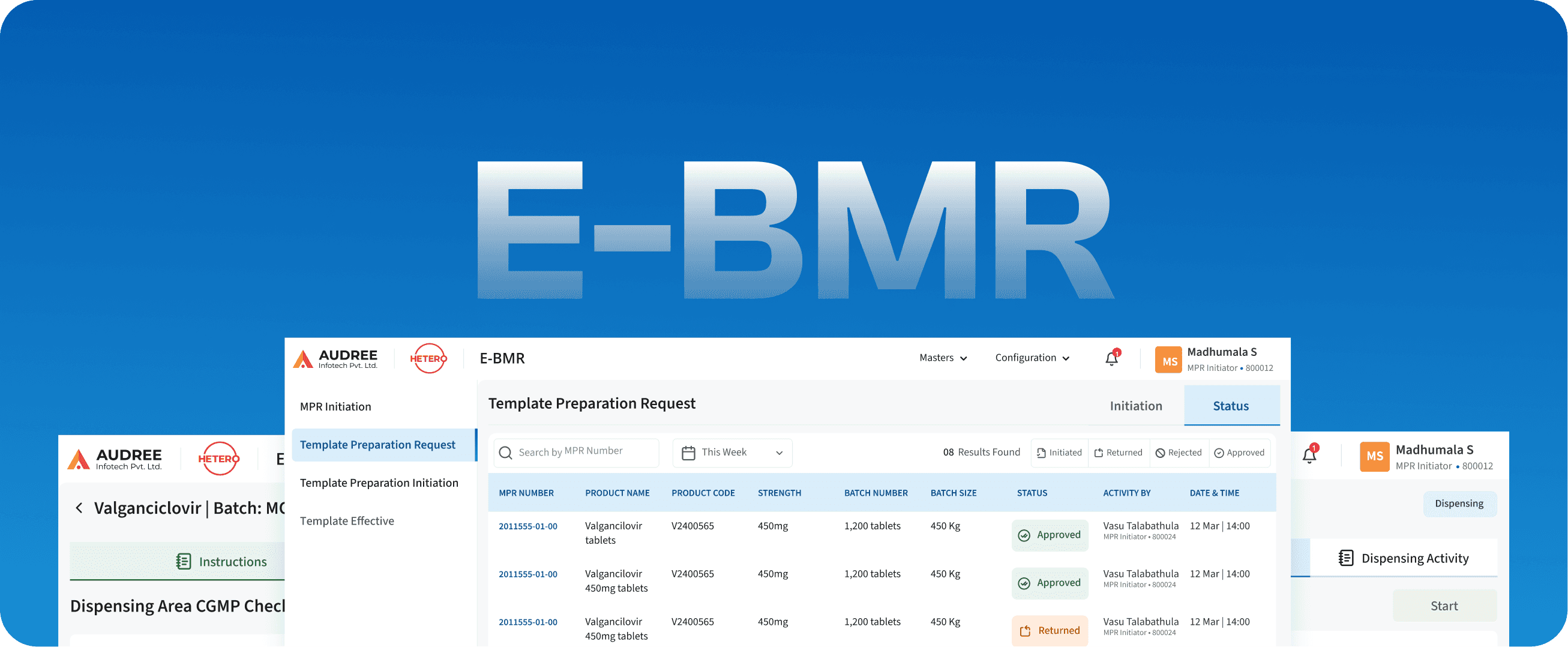

Batch Manufacturing Recall
WMPS


Warehouse Management System
DMS
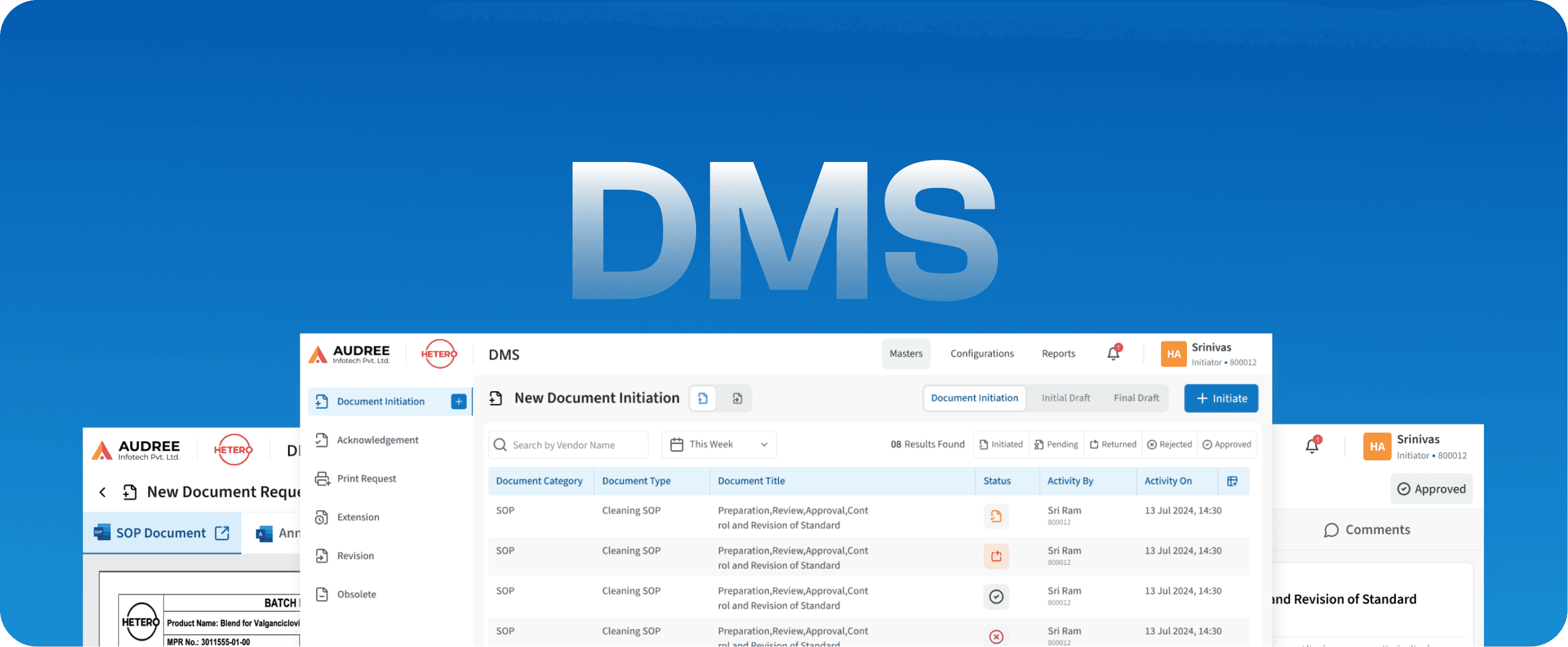

Document Management System
CAPA


Corrective And Preventive Actions
QAS


Quality Agreement System
Vendor Portal
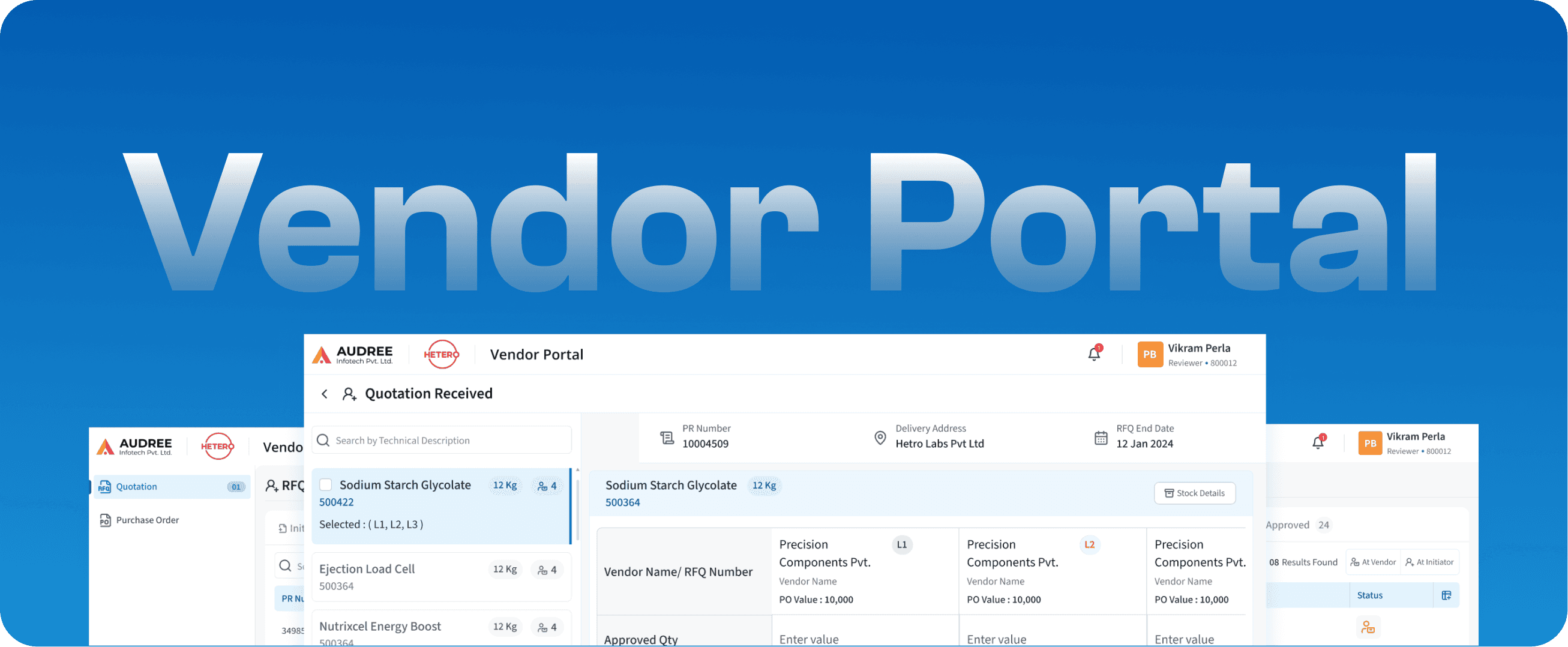

Vendor Management System
LIR-AER
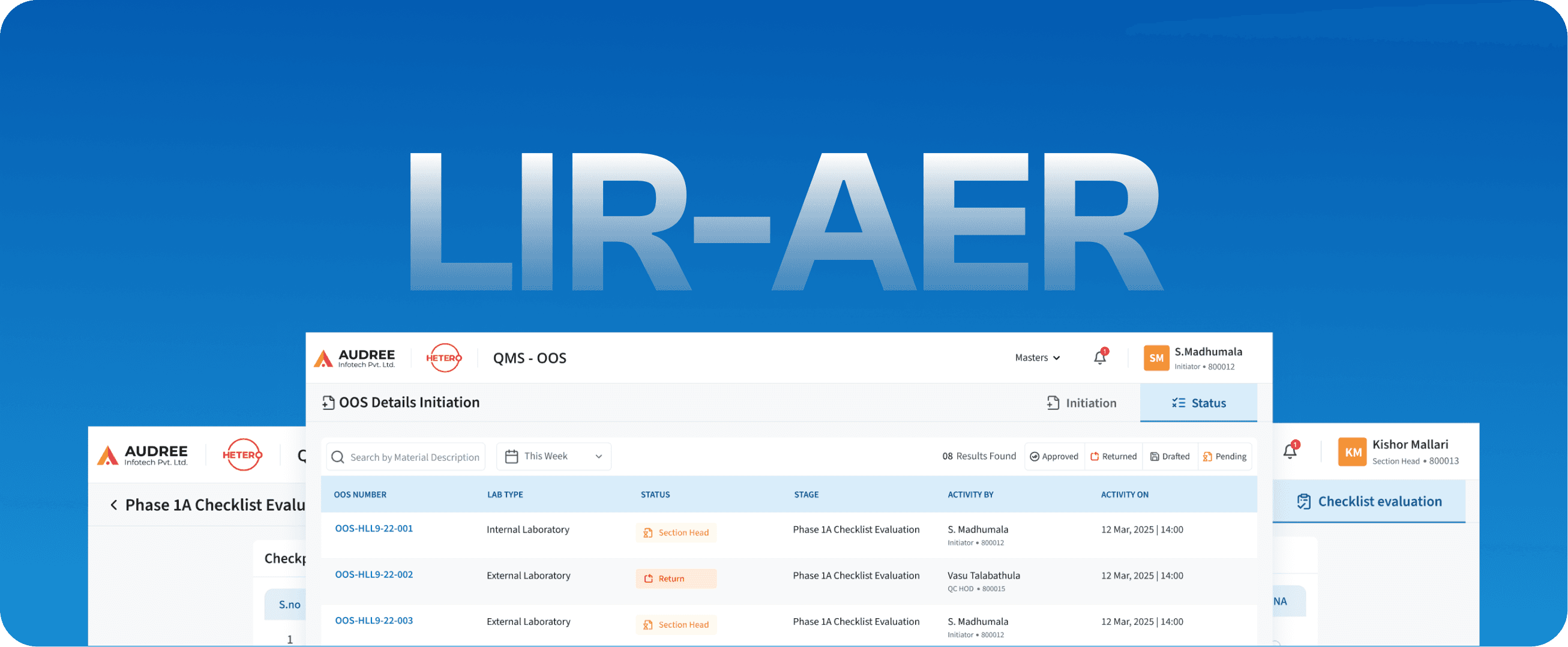

Laboratory Information Record
OOS
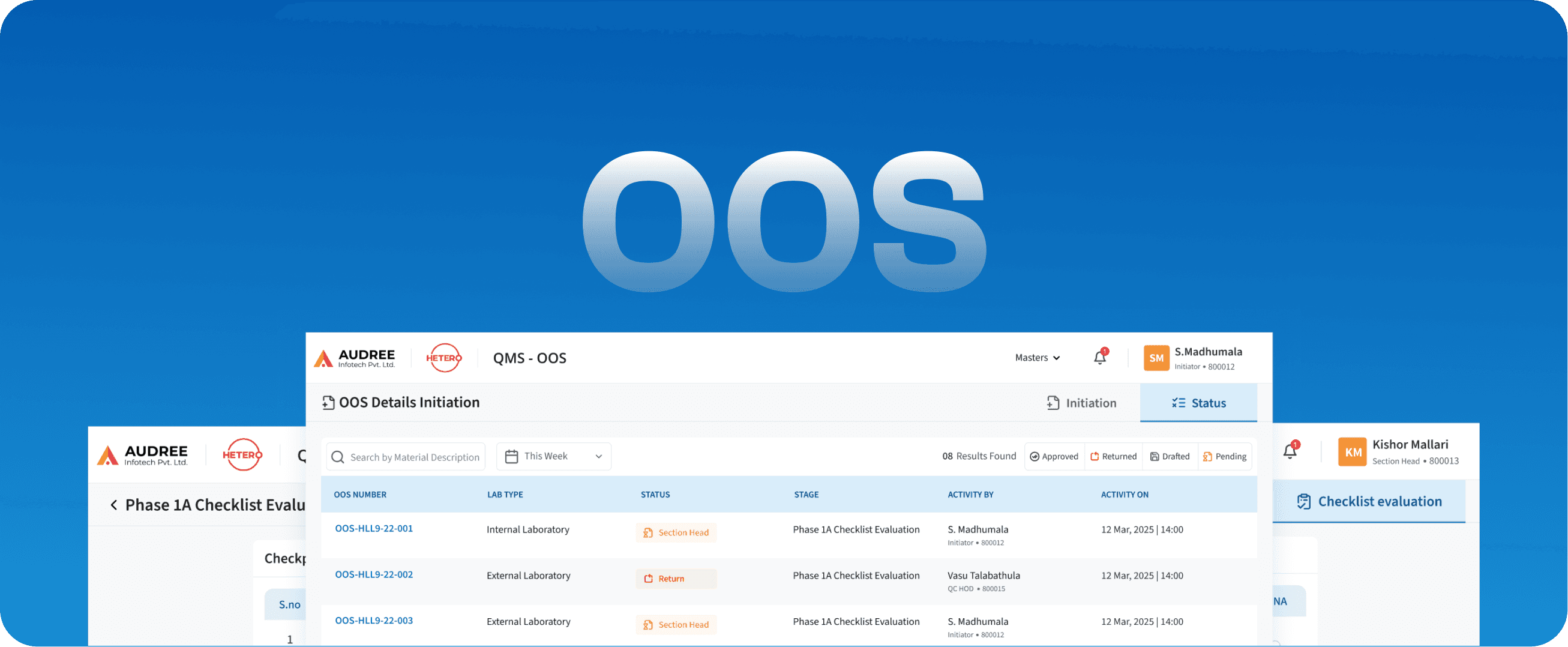

Out Of Specification
CMS
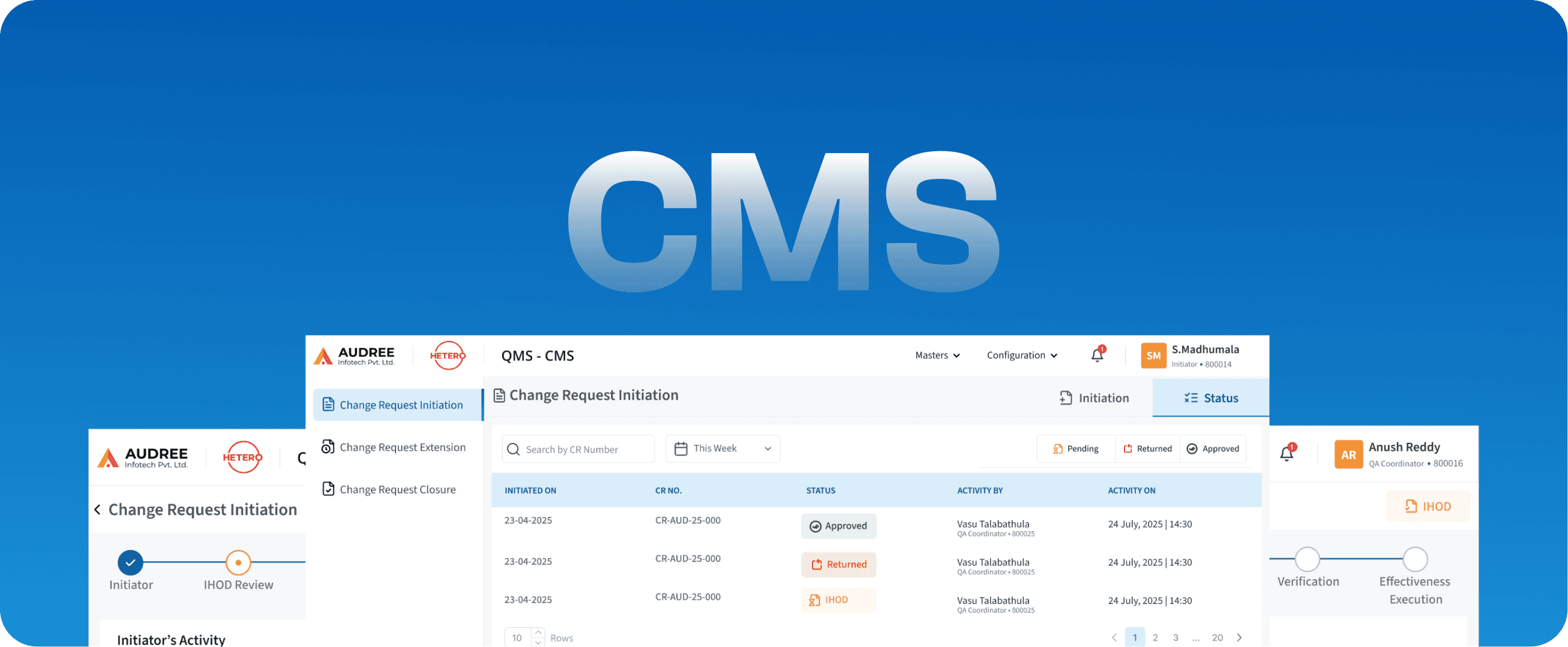

Change Management System
IMS
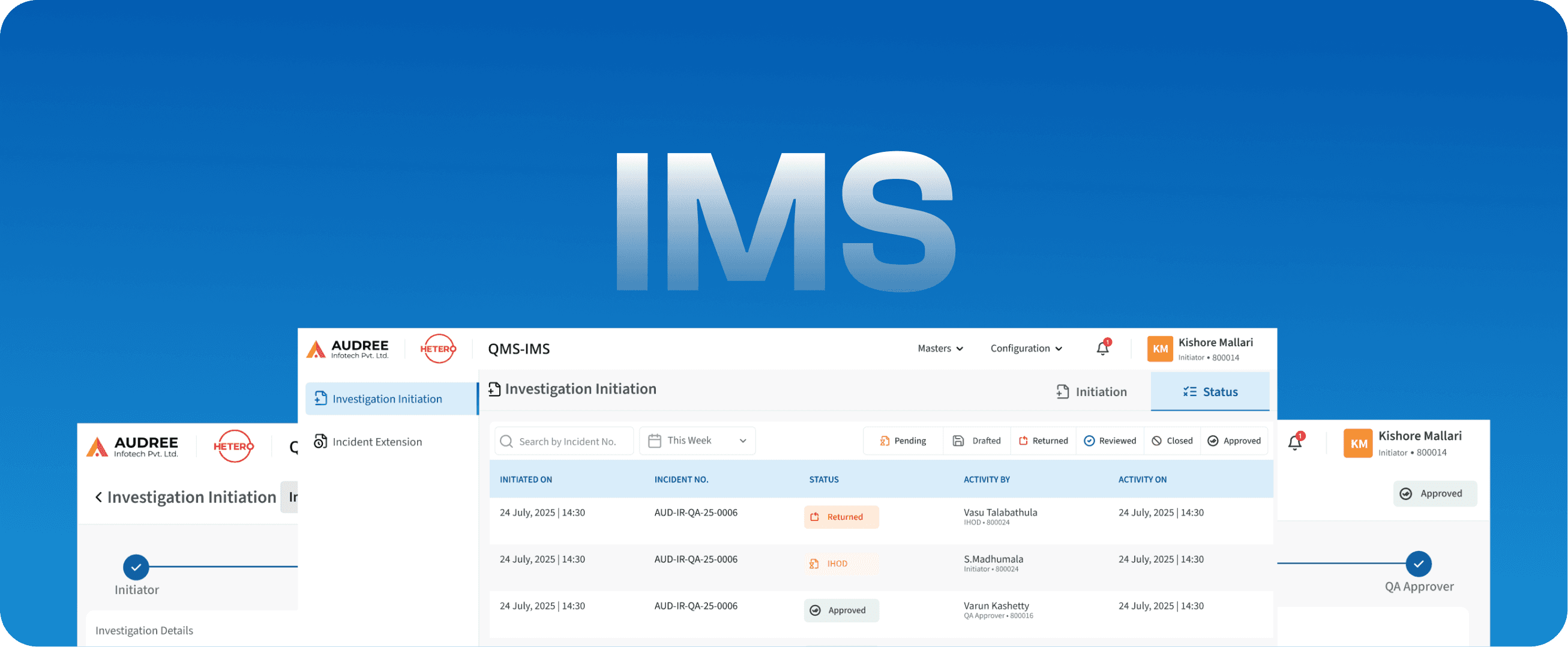

Incident Management System
BRMS-API
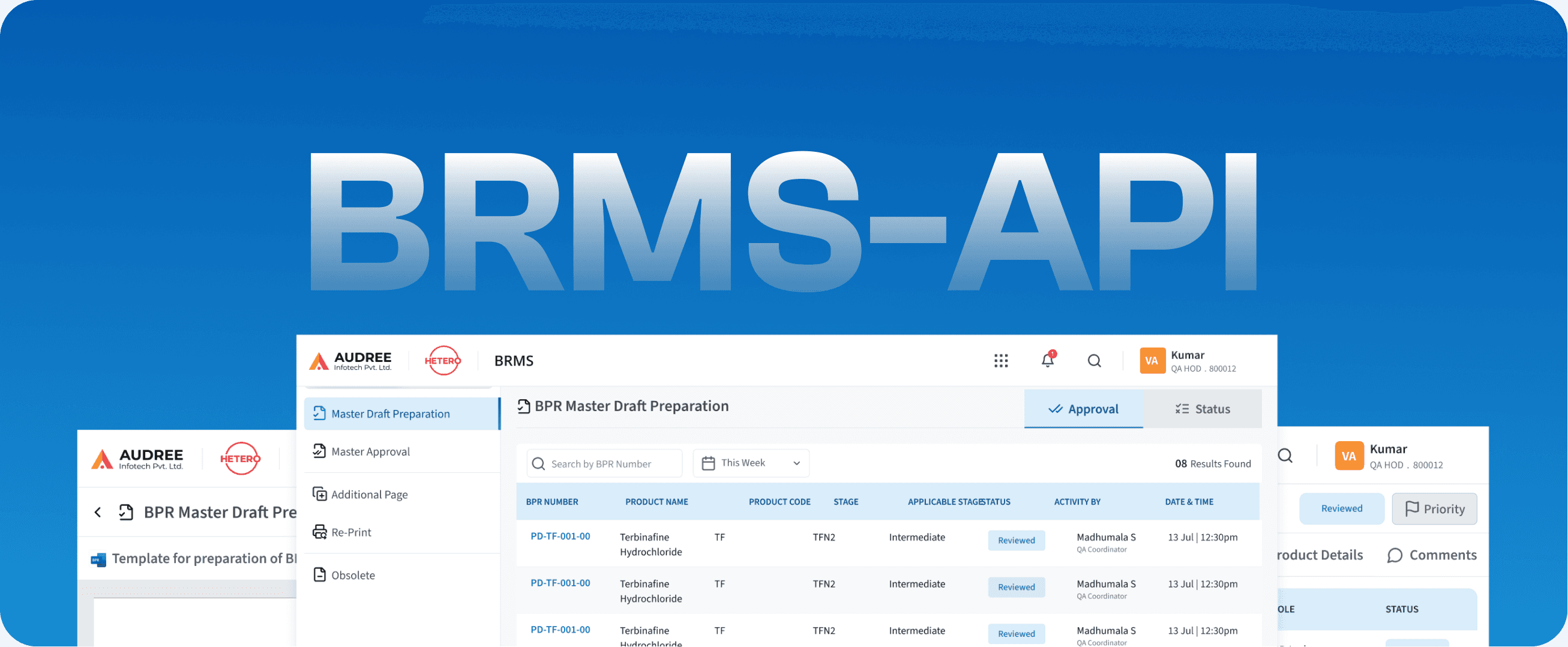

Batch Record- Active Pharmaceutical Ingredient
E-BRMS
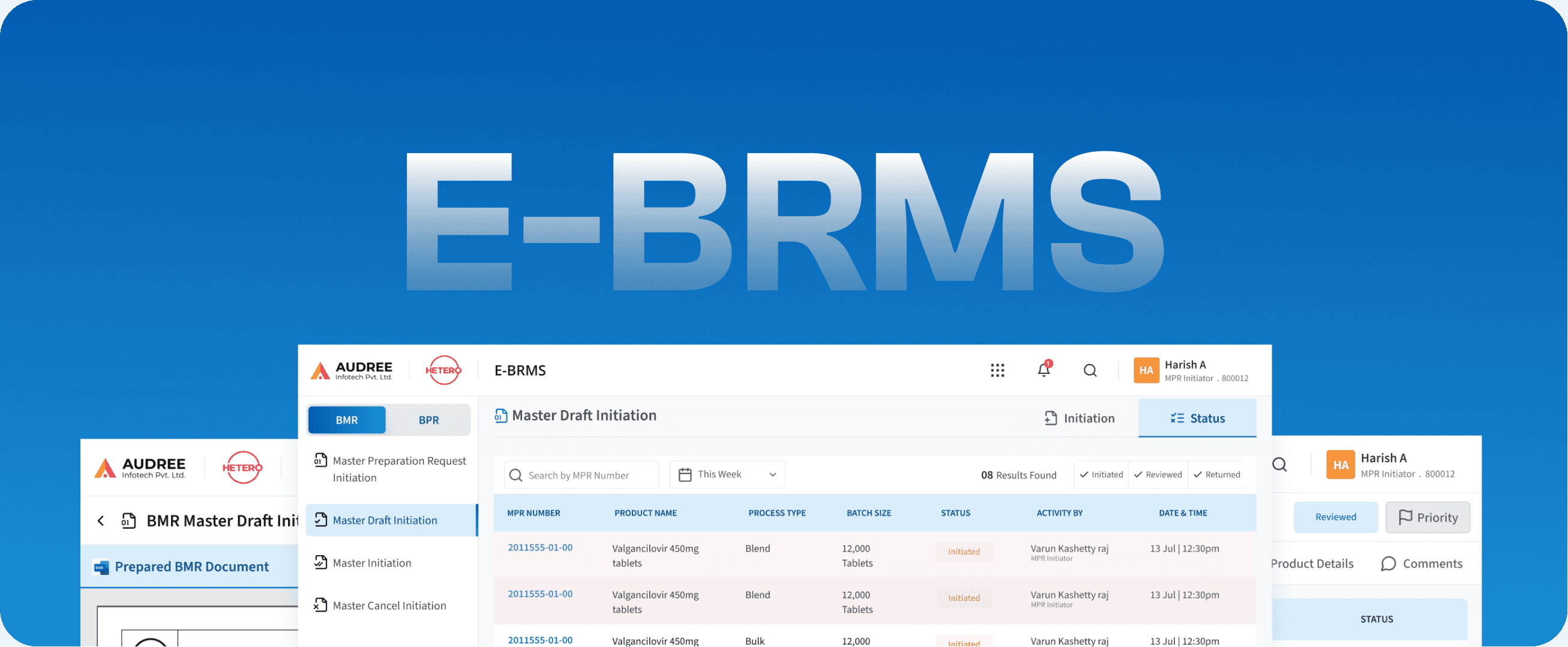

Batch Record Management System
RIMS
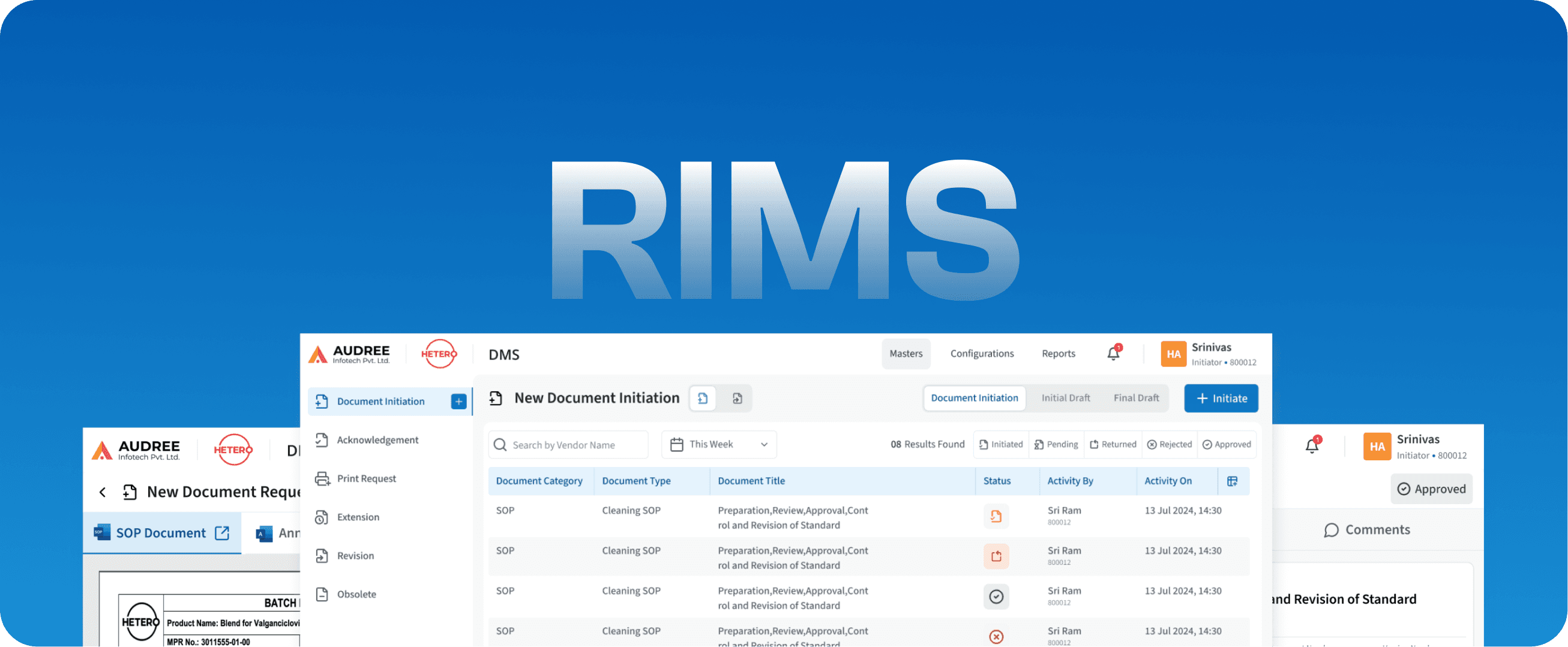

Regulatory Information Management System
APQR
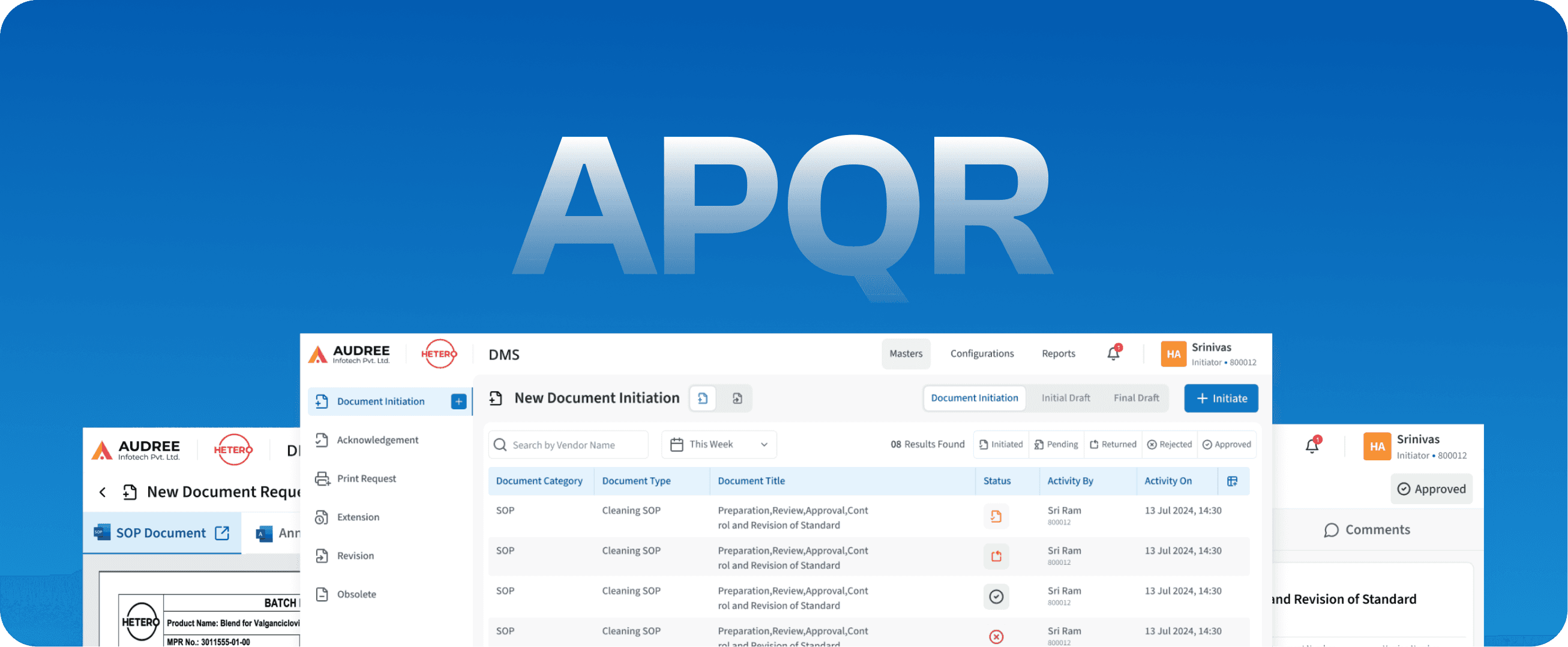

Annual Product Quality Review
RCAI

Root Cause Analysis with Intelligence
LMS

Learning Management System
LIMS

Laboratory Information Management System
S & OP

Sales & Operations Planning
E-BMR

Batch Manufacturing Recall
RIMS
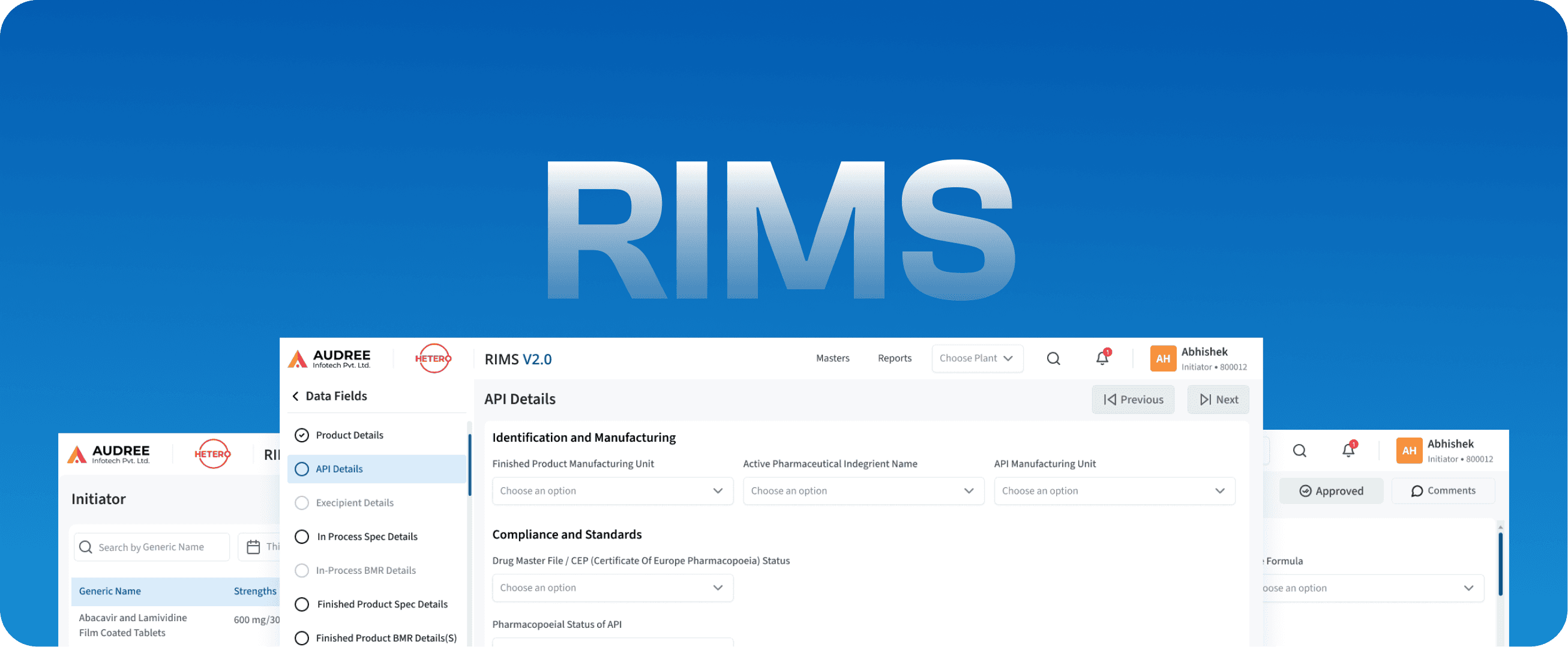
Regulatory Information Management Systems
IMS

Incident Management System
BRMS-API

Batch Record- Active Pharmaceutical Ingredient
E-BRMS

Batch Record Management System
APQR

Annual Product Quality Review
RIMS

Regulatory Information Management System
WMPS

Warehouse Management System
DMS

Document Management System
CMS

Change Management System
OOS

Out Of Specification
LIR-AER

Laboratory Information Record
Vendor Portal

Vendor Management System
QAS

Quality Agreement System
CAPA

Corrective And Preventive Actions

