Redefining Batch Records From Complexity to Clarity
Redefining Batch Records From Complexity to Clarity
Redefining Batch Records From Complexity to Clarity
About Project
About Project
About Project
The Batch Manufacturing Record (BMR) module manages the full lifecycle of manufacturing documentation from creation and review to approval, issuance, and retrieval. It’s a critical system in pharma, ensuring every manufacturing step is properly recorded, controlled, and compliant.
The earlier BMR system had an higher task completion time, scattered workflows, and unclear lifecycle stages, making it difficult for teams to create, manage, and trace BMRs efficiently.
To simplify BMR creation and lifecycle management with a modern, structured, and traceable workflow that reduces confusion and makes the entire process faster and more compliant.
The Batch Manufacturing Record (BMR) module manages the full lifecycle of manufacturing documentation from creation and review to approval, issuance, and retrieval. It’s a critical system in pharma, ensuring every manufacturing step is properly recorded, controlled, and compliant.
The earlier BMR system had an higher task completion time, scattered workflows, and unclear lifecycle stages, making it difficult for teams to create, manage, and trace BMRs efficiently.
To simplify BMR creation and lifecycle management with a modern, structured, and traceable workflow that reduces confusion and makes the entire process faster and more compliant.
About Project
Pharmaceutical
Team
Anush Reddy, S.Madhumala
Subscription Category
Quick win
Project start Year
August 2024
Core Business Challenges
Core Business Challenges
Core Business Challenges
Poor User Adoption Due to Outdated Experience
Poor User Adoption Due to Outdated Experience
Poor User Adoption Due to Outdated Experience
The outdated interface made it difficult to find key sections or track changes, leading to low engagement and heavy reliance on offline tools.
The outdated interface made it difficult to find key sections or track changes, leading to low engagement and heavy reliance on offline tools.
The outdated interface made it difficult to find key sections or track changes, leading to low engagement and heavy reliance on offline tools.
Stuck in a Loop, Slowed by Inefficiency
Stuck in a Loop, Slowed by Inefficiency
Stuck in a Loop, Slowed by Inefficiency
Repeated support tickets piled up as scattered forms and unclear grouping made BMR updates slow and confusing.
Repeated support tickets piled up as scattered forms and unclear grouping made BMR updates slow and confusing.
Repeated support tickets piled up as scattered forms and unclear grouping made BMR updates slow and confusing.
Heavy Dependence on Manual Coordination
Heavy Dependence on Manual Coordination
Reviewers depended on emails, calls, and spreadsheets for clarifications, creating delays, confusion, and poor traceability.
Reviewers depended on emails, calls, and spreadsheets for clarifications, creating delays, confusion, and poor traceability.
Heavy Dependence on Manual Coordination
Reviewers depended on emails, calls, and spreadsheets for clarifications, creating delays, confusion, and poor traceability.
Our Approach
Our Approach
Our Approach
Mapping User Flows to Uncover Hidden Gaps
We studied how Production, QA, and Documentation teams actually create BMRs, revealing repeated inputs, unclear transitions, and heavy offline coordination. This gave us a clear roadmap for a guided, streamlined flow.
Mapping User Flows to Uncover Hidden Gaps
We studied how Production, QA, and Documentation teams actually create BMRs, revealing repeated inputs, unclear transitions, and heavy offline coordination. This gave us a clear roadmap for a guided, streamlined flow.
Mapping User Flows to Uncover Hidden Gaps
We studied how Production, QA, and Documentation teams actually create BMRs, revealing repeated inputs, unclear transitions, and heavy offline coordination. This gave us a clear roadmap for a guided, streamlined flow.
Turning Complex Batch Records Into a Clear, Structured Flow
Turning Complex Batch Records Into a Clear, Structured Flow
Turning Complex Batch Records Into a Clear, Structured Flow
We rebuilt the entire BMR lifecycle into a guided, stage-wise flow that reflects real manufacturing practices. Manual decisions and backtracking were removed, reviews became predictable with clear version cues, and a Manufacturing Packing tab switch lets users move between flows effortlessly.
We rebuilt the entire BMR lifecycle into a guided, stage-wise flow that reflects real manufacturing practices. Manual decisions and backtracking were removed, reviews became predictable with clear version cues, and a Manufacturing Packing tab switch lets users move between flows effortlessly.




One Interface, Two Workflows
One Interface, Two Workflows
One Interface, Two Workflows
Since BMR and BPR share the same structure but differ in function, we added a simple tab switch to toggle between them. This keeps both workflows clearly separated while using one familiar layout—reducing duplication, avoiding confusion, and helping teams switch contexts effortlessly.
Since BMR and BPR share the same structure but differ in function, we added a simple tab switch to toggle between them. This keeps both workflows clearly separated while using one familiar layout—reducing duplication, avoiding confusion, and helping teams switch contexts effortlessly.
Since BMR and BPR share the same structure but differ in function, we added a simple tab switch to toggle between them. This keeps both workflows clearly separated while using one familiar layout—reducing duplication, avoiding confusion, and helping teams switch contexts effortlessly.
One Unified View for Confident Review
One Unified View for Confident Review
One Unified View for Confident Review
The review screen brings everything into one workspace the BMR document on the left and all product details and comments on the right.
Reviewers no longer switch tabs or hunt for information. Key details are visible without scrolling, making verification faster and more accurate. Reducing back-and-forth and simplifying the entire review process.
The review screen brings everything into one workspace the BMR document on the left and all product details and comments on the right.
Reviewers no longer switch tabs or hunt for information. Key details are visible without scrolling, making verification faster and more accurate. Reducing back-and-forth and simplifying the entire review process.
The review screen brings everything into one workspace the BMR document on the left and all product details and comments on the right.
Reviewers no longer switch tabs or hunt for information. Key details are visible without scrolling, making verification faster and more accurate. Reducing back-and-forth and simplifying the entire review process.




Priority Made Visible. Decisions Made Faster.
Priority Made Visible. Decisions Made Faster.
Priority Made Visible. Decisions Made Faster.
The older BMR workflow made it difficult for teams to identify which records needed immediate attention.
We added a Priority Selector so teams can instantly see which BMRs need urgent attention. Users can set or adjust priority anytime, ensuring critical batches never get lost in the workflow.
The older BMR workflow made it difficult for teams to identify which records needed immediate attention.
We added a Priority Selector so teams can instantly see which BMRs need urgent attention. Users can set or adjust priority anytime, ensuring critical batches never get lost in the workflow.
The older BMR workflow made it difficult for teams to identify which records needed immediate attention.
We added a Priority Selector so teams can instantly see which BMRs need urgent attention. Users can set or adjust priority anytime, ensuring critical batches never get lost in the workflow.
Design guided by Visual Clarity
Design guided by Visual Clarity
Design guided by Visual Clarity
Smart UI cues like status tags, icons, empty states, and comment cards make every step easy to understand. Users instantly see what’s pending, completed, or needs attention.
Smart UI cues like status tags, icons, empty states, and comment cards make every step easy to understand. Users instantly see what’s pending, completed, or needs attention.












A Modern UI for a Smarter Workflow
A Modern UI for a Smarter Workflow
A Modern UI for a Smarter Workflow
The redesigned UI brings clarity to every screen with structured layouts, cleaner hierarchies, and components that make actions easy and predictable.
The redesigned UI brings clarity to every screen with structured layouts, cleaner hierarchies, and components that make actions easy and predictable.



Result That Transformed Batch Manufacturing Lifecycle
Result That Transformed Batch Manufacturing Lifecycle
Result That Transformed Batch Manufacturing Lifecycle
The redesigned BMR system completely transformed how teams create and issue batch records. What was once a slow, document-heavy, and error-prone is now a guided, traceable process.
The redesigned BMR system completely transformed how teams create and issue batch records. What was once a slow, document-heavy, and error-prone is now a guided, traceable process.

Stronger Compliance & Traceability
Stronger Compliance & Traceability
Stronger Compliance & Traceability
Built-in validations, version history, and approval tracking ensured every batch record change was accurate, traceable, and audit-ready.
Built-in validations, version history, and approval tracking ensured every batch record change was accurate, traceable, and audit-ready.
Built-in validations, version history, and approval tracking ensured every batch record change was accurate, traceable, and audit-ready.

Faster, Error-Free Execution
Clear record structures, built-in validations, and step-by-step flows reduced manual referencing, minimized errors, and shortened review cycles.

Scalable Global Adoption
The redesigned BRMS scaled from limited use to a trusted platform adopted across global pharma plants, ensuring consistent and accurate batch record creation.

Faster, Error-Free Execution
Faster, Error-Free Execution
Clear record structures, built-in validations, and step-by-step flows reduced manual referencing, minimized errors, and shortened review cycles.
Clear record structures, built-in validations, and step-by-step flows reduced manual referencing, minimized errors, and shortened review cycles.

Scalable Global Adoption
Scalable Global Adoption
The redesigned BRMS scaled from limited use to a trusted platform adopted across global pharma plants, ensuring consistent and accurate batch record creation.
The redesigned BRMS scaled from limited use to a trusted platform adopted across global pharma plants, ensuring consistent and accurate batch record creation.
Deep-Dive Into More System
Deep-Dive Into More System
Deep-Dive Into More System
Browse every optimised Software and explore how legacy systems became intuitive.
Browse every optimised Software and explore how legacy systems became intuitive.
Browse every optimised Software and explore how legacy systems became intuitive.
RCAI

Root Cause Analysis with Intelligence
LMS
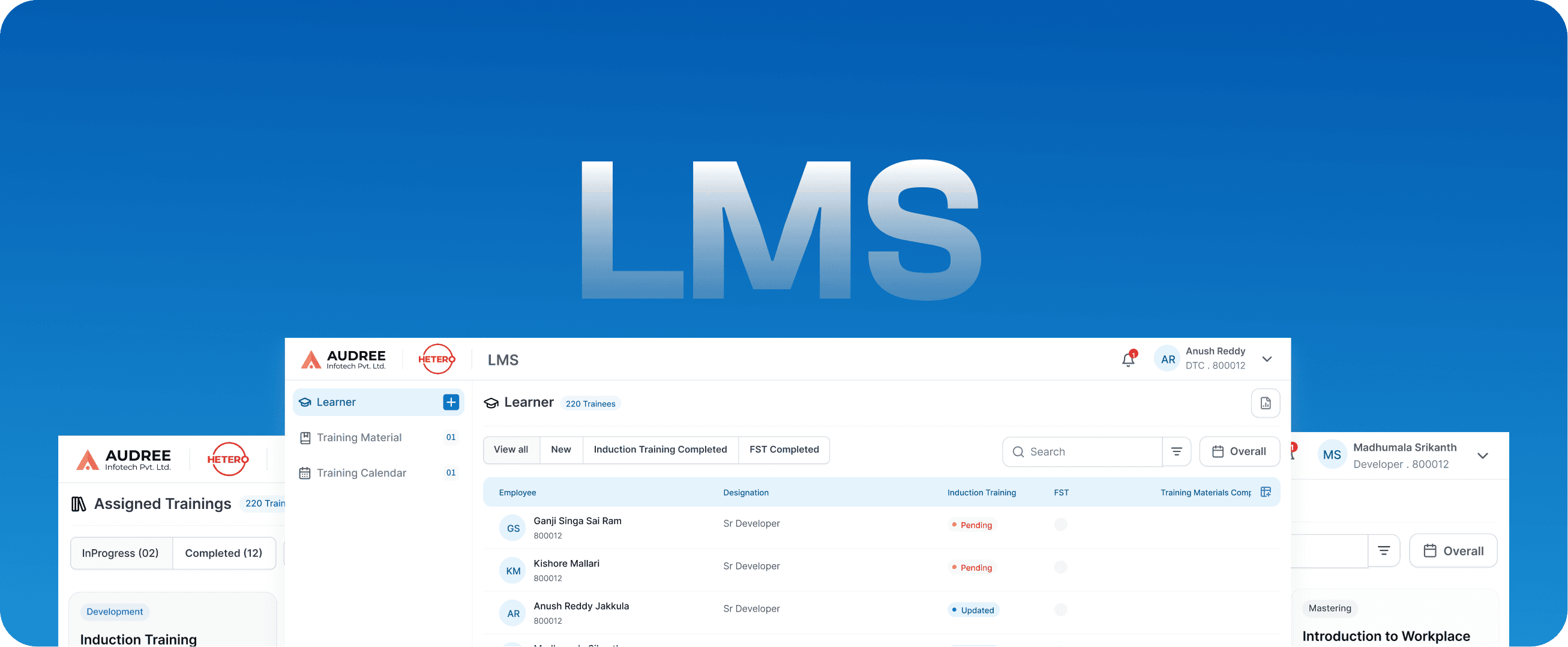
Learning Management System
LIMS
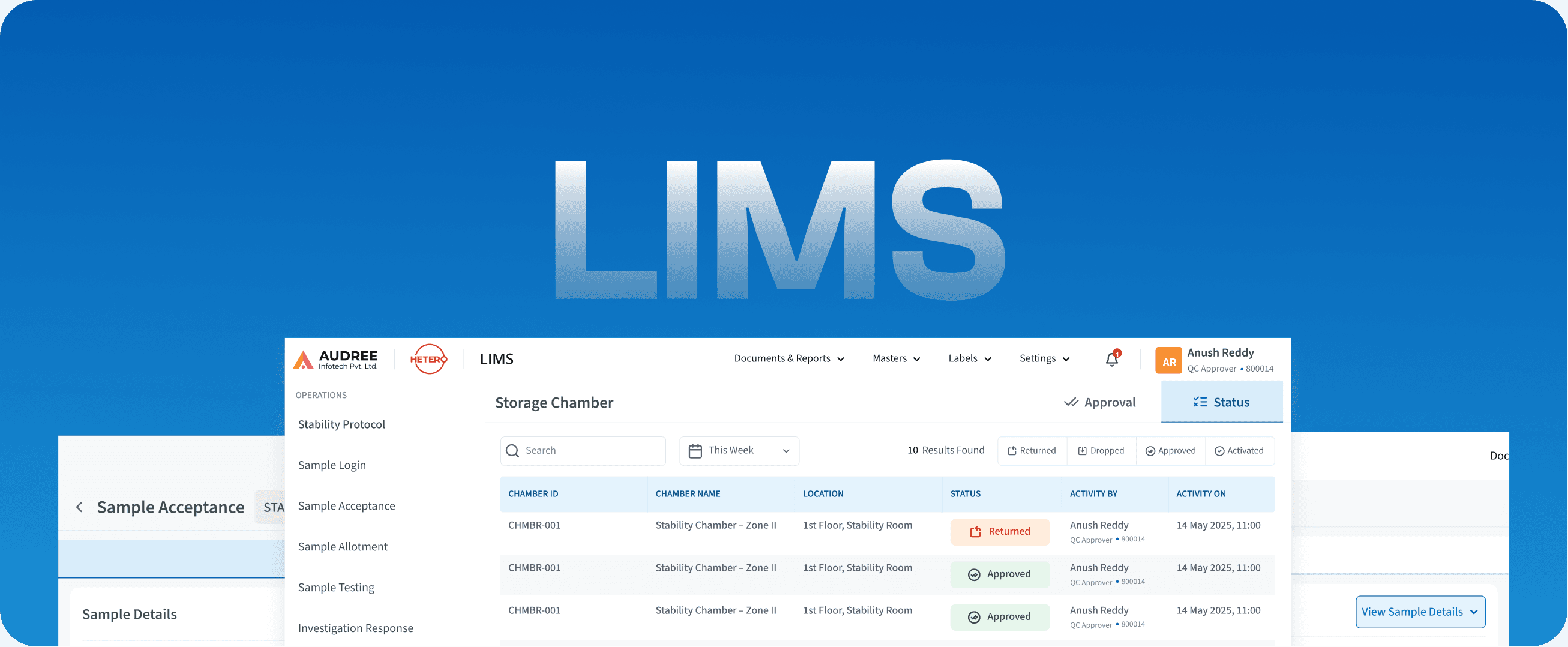
Laboratory Information Management System
S & OP
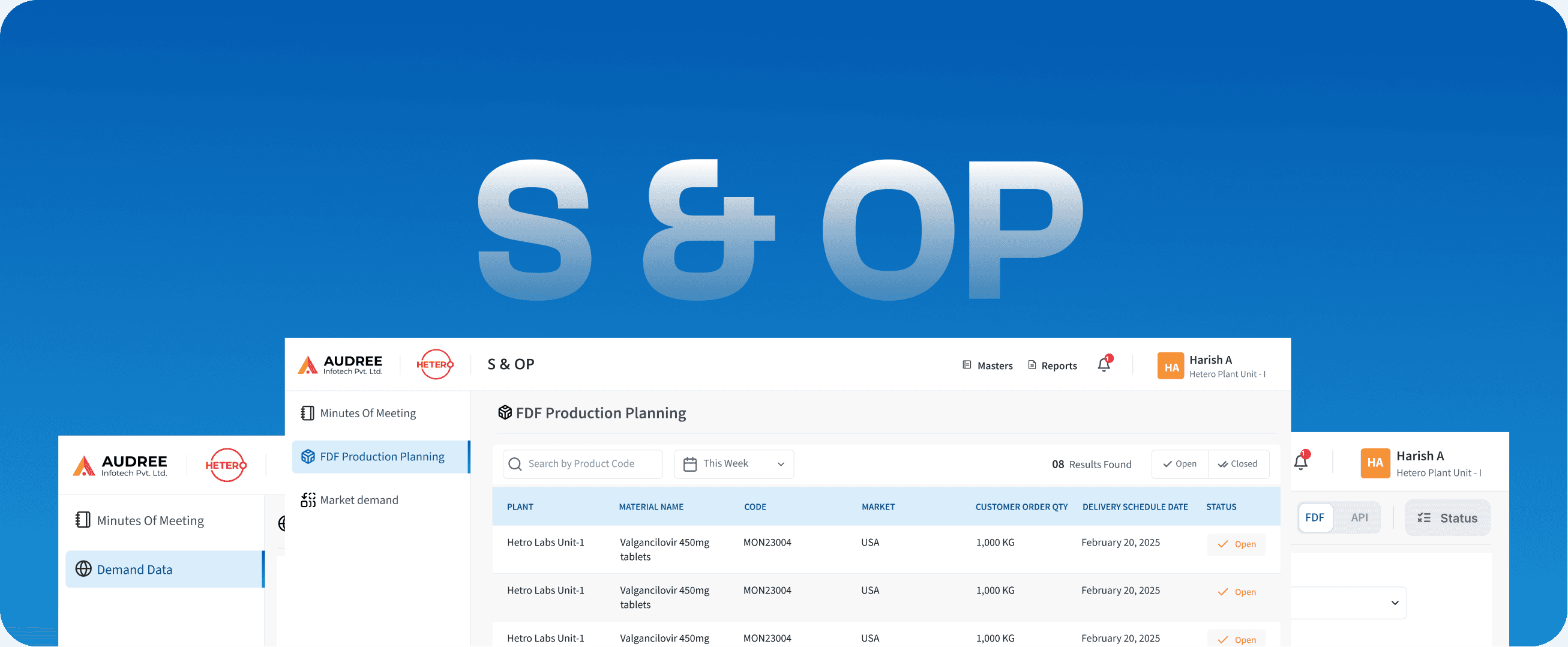
Sales & Operations Planning
E-BMR
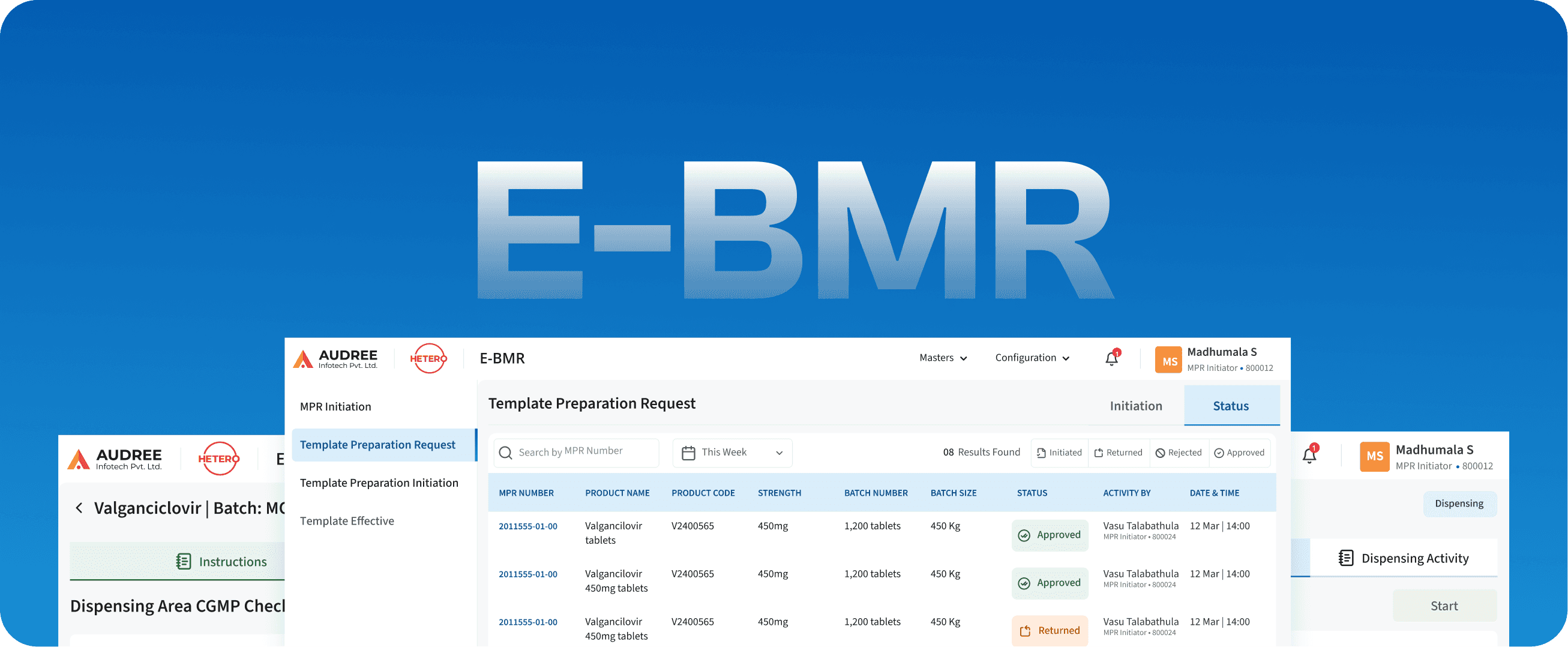
Batch Manufacturing Recall
WMPS

Warehouse Management System
DMS
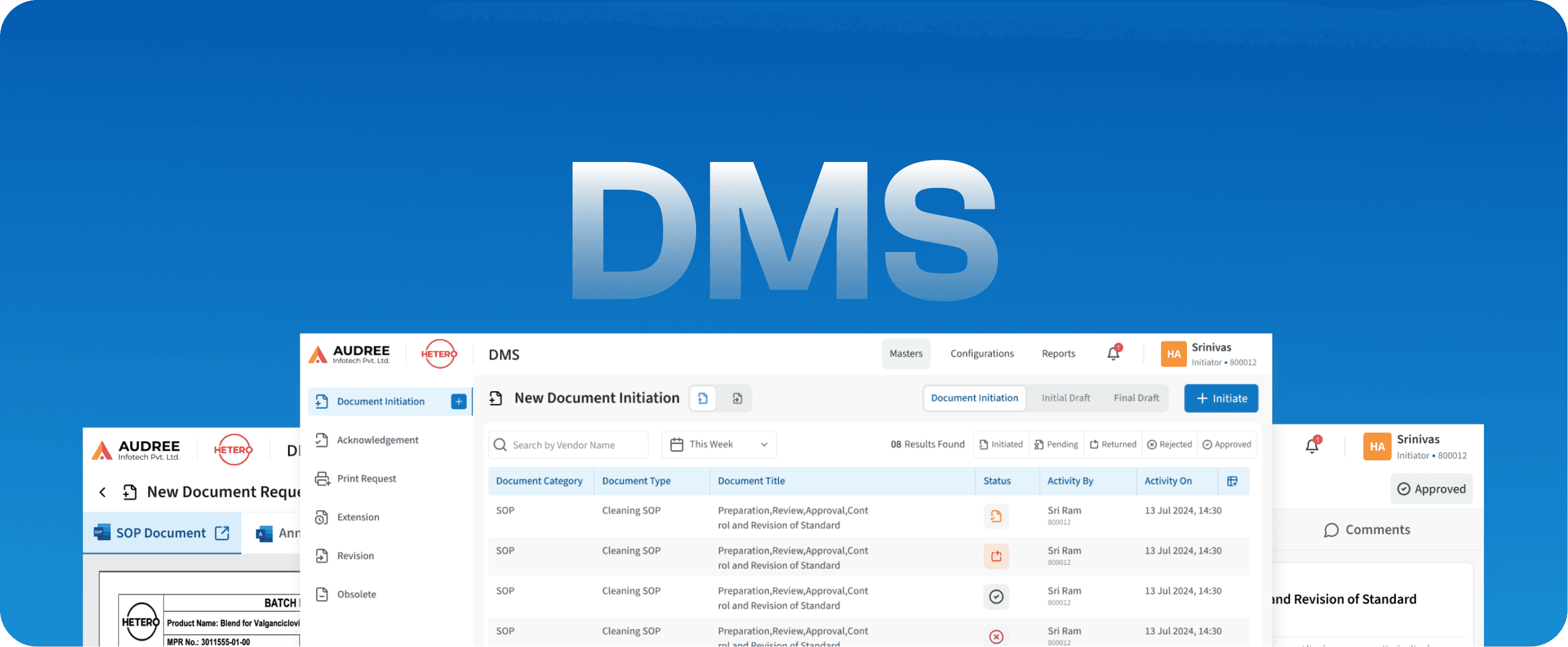
Document Management System
CAPA

Corrective And Preventive Actions
QAS

Quality Agreement System
Vendor Portal
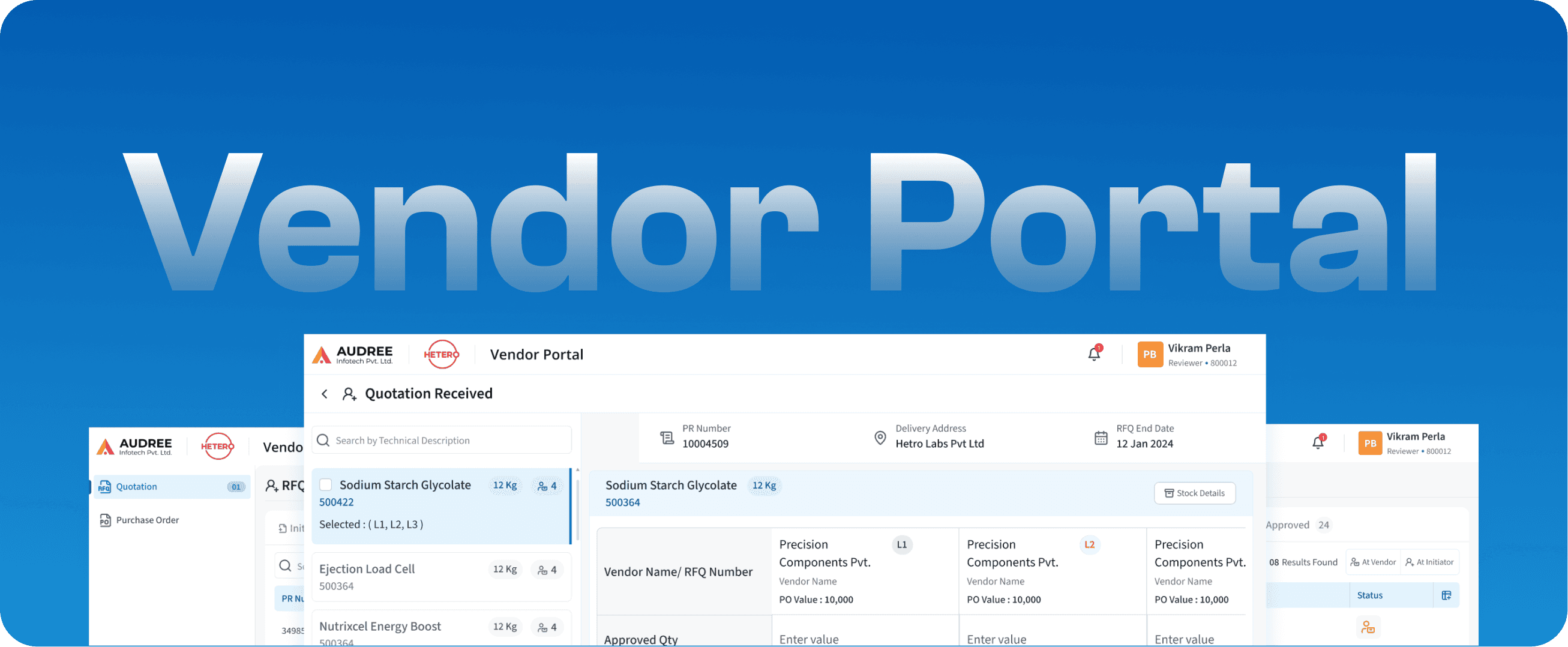
Vendor Management System
LIR-AER
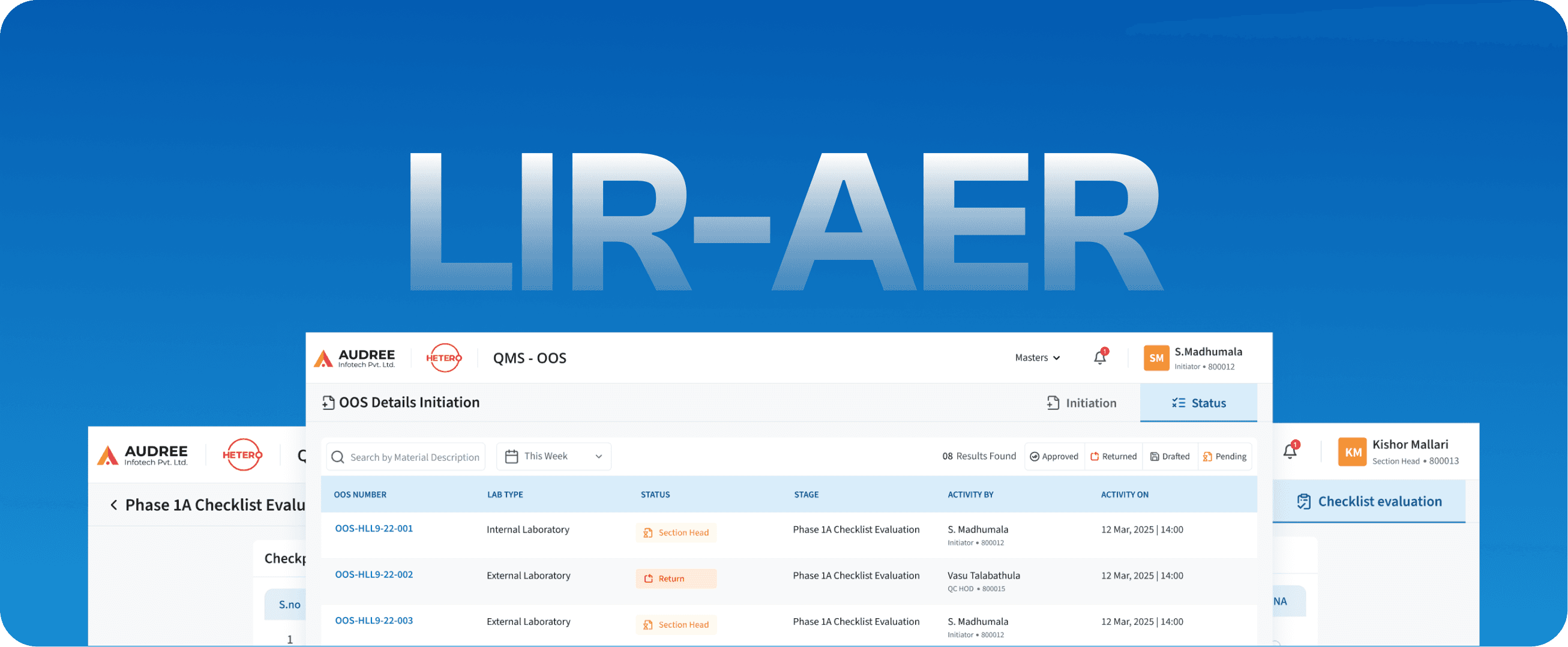
Laboratory Information Record
OOS
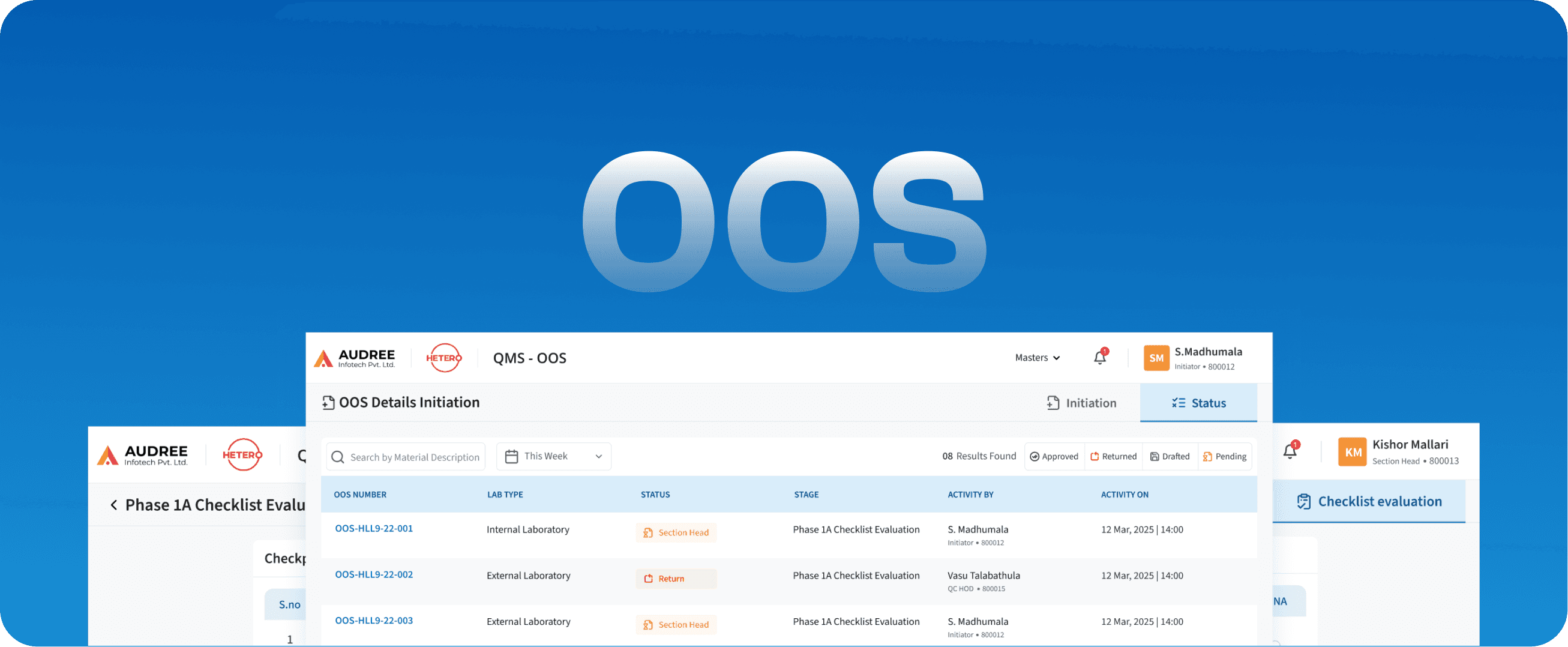
Out Of Specification
CMS
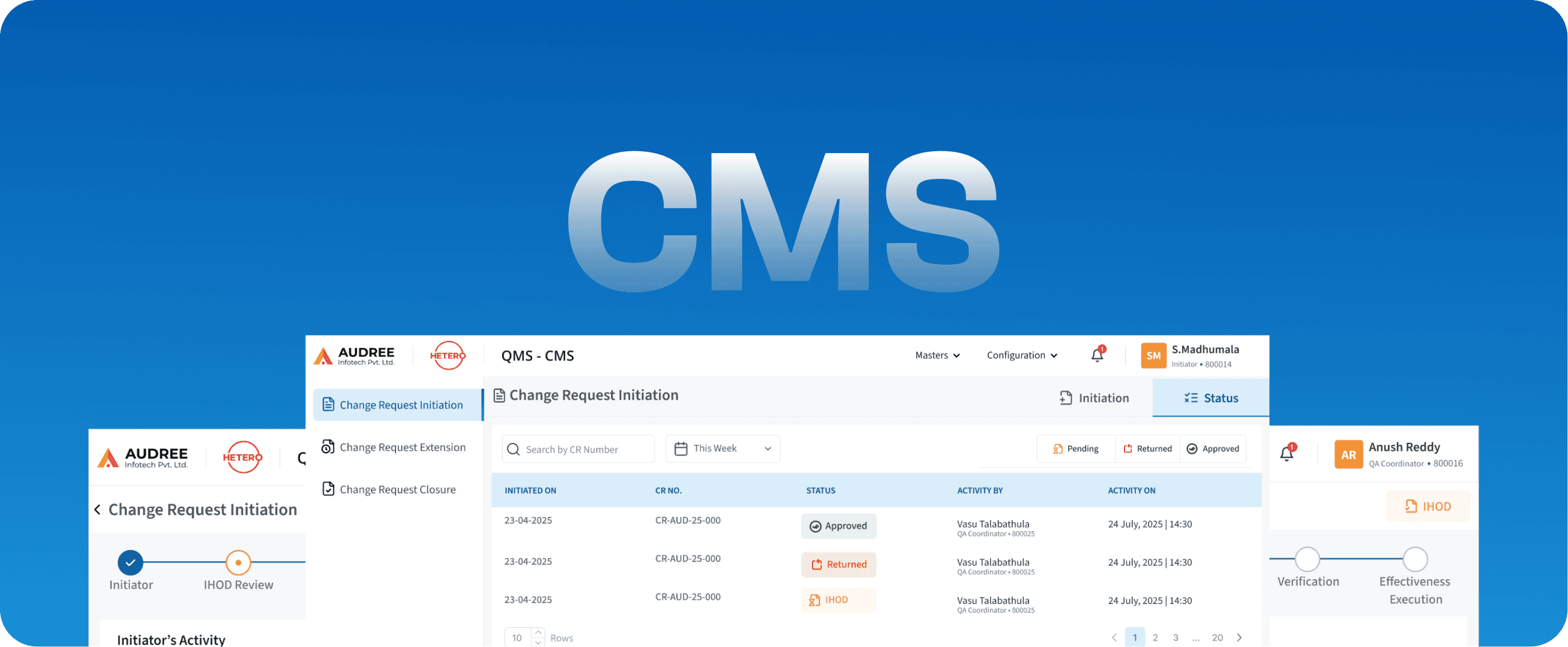
Change Management System
IMS
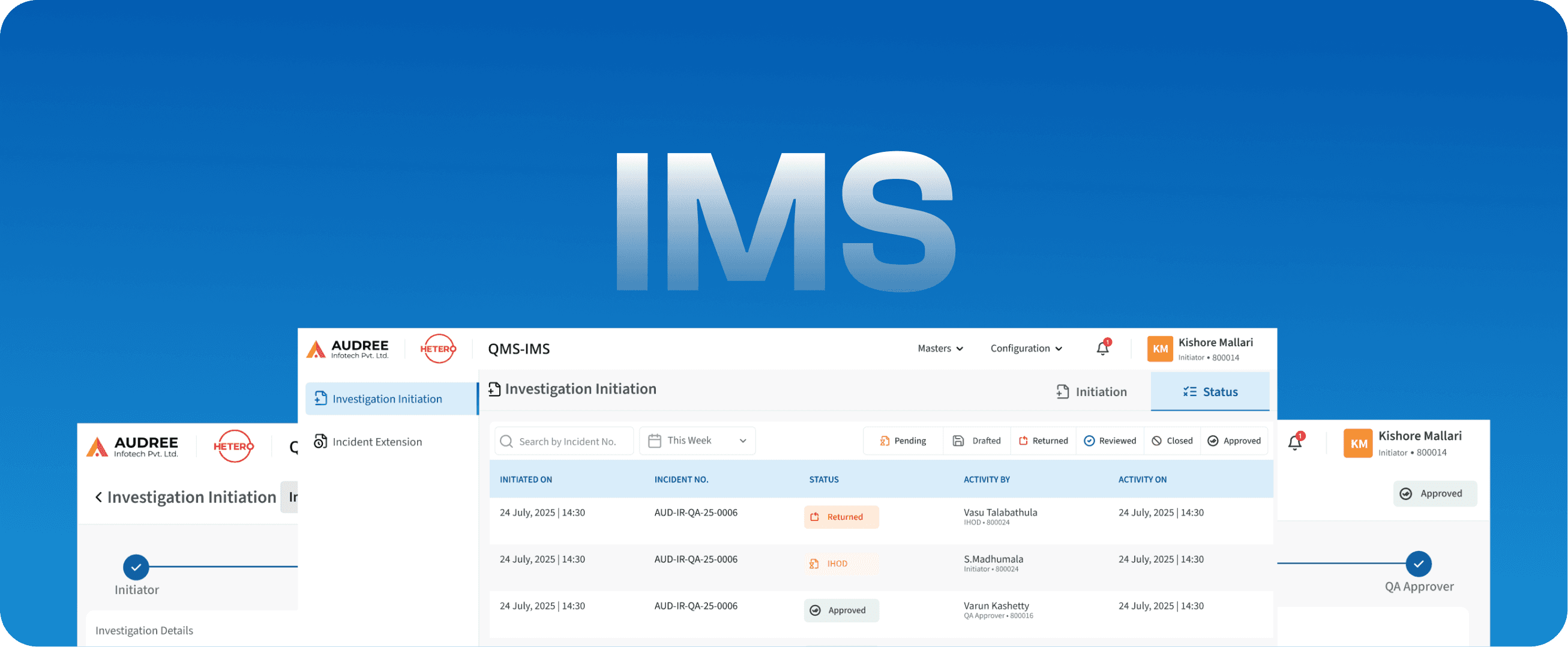
Incident Management System
BRMS-API
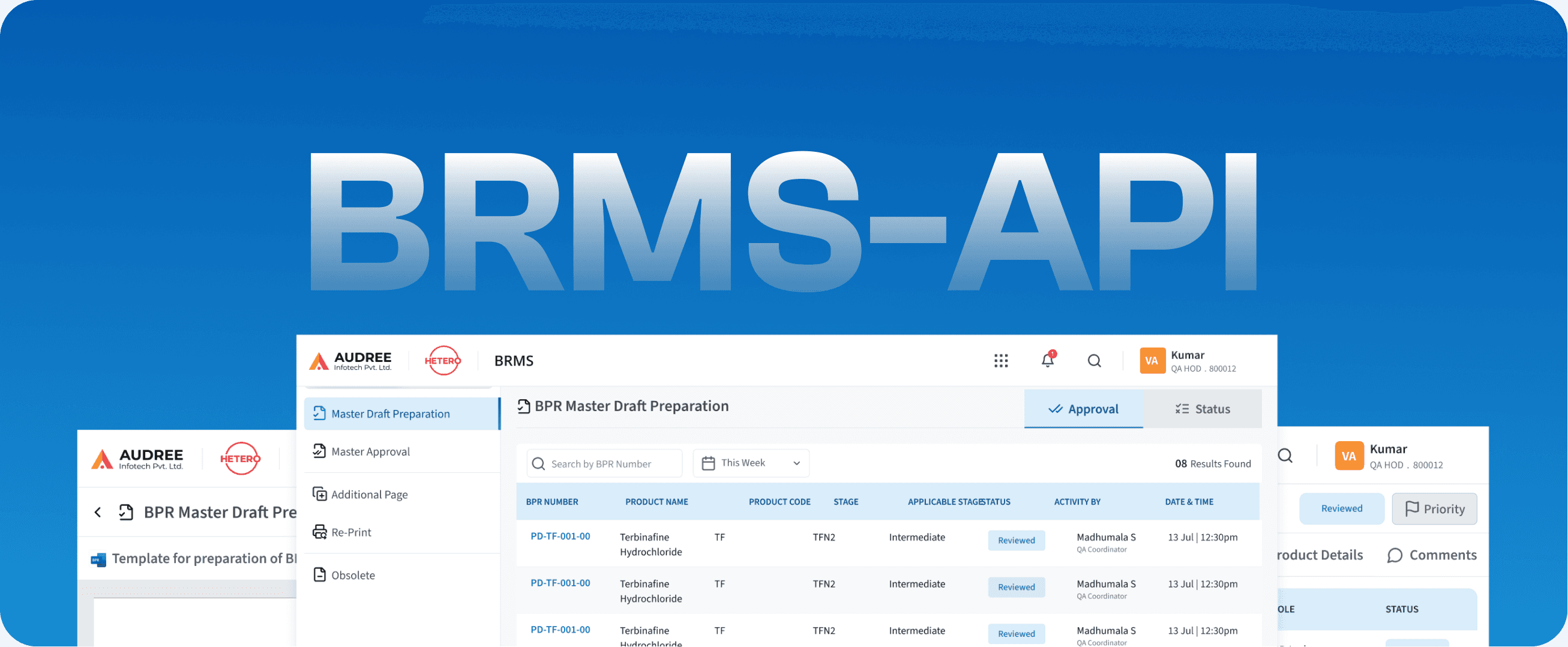
Batch Record- Active Pharmaceutical Ingredient
E-BRMS
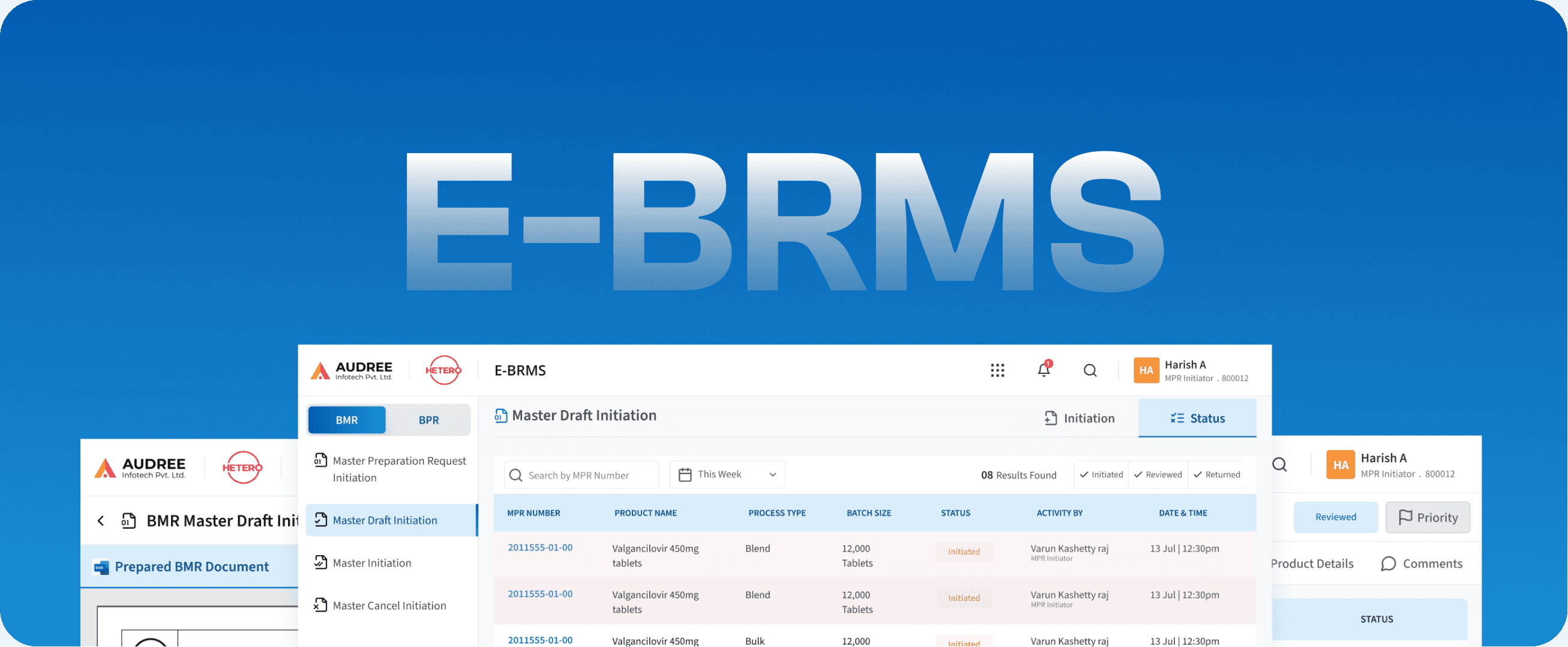
Batch Record Management System
RIMS
Regulatory Information Management System
APQR
Annual Product Quality Review
RCAI

Root Cause Analysis with Intelligence
LMS

Learning Management System
LIMS

Laboratory Information Management System
S & OP

Sales & Operations Planning
E-BMR

Batch Manufacturing Recall
RIMS
Regulatory Information Management Systems
IMS

Incident Management System
BRMS-API

Batch Record- Active Pharmaceutical Ingredient
E-BRMS

Batch Record Management System
APQR

Annual Product Quality Review
RIMS

Regulatory Information Management System
WMPS

Warehouse Management System
DMS

Document Management System
CMS

Change Management System
OOS

Out Of Specification
LIR-AER

Laboratory Information Record
Vendor Portal

Vendor Management System
QAS

Quality Agreement System
CAPA

Corrective And Preventive Actions

